J Korean Acad Conserv Dent.
2007 Mar;32(2):151-161. 10.5395/JKACD.2007.32.2.151.
The effect of the pH of remineralized buffer solutions on dentin remineralization
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Yonsei University, Korea. chanyoungl@yumc.yonsei.ac.kr
- KMID: 2175848
- DOI: http://doi.org/10.5395/JKACD.2007.32.2.151
Abstract
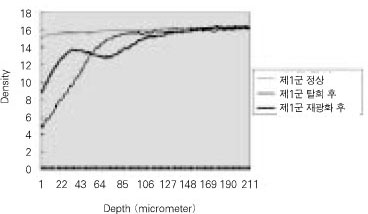
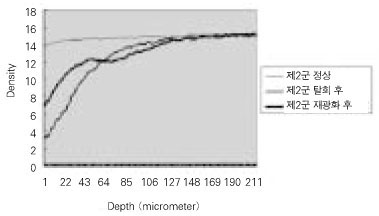
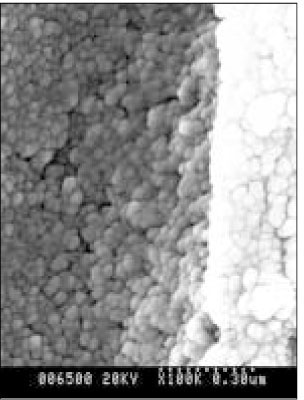
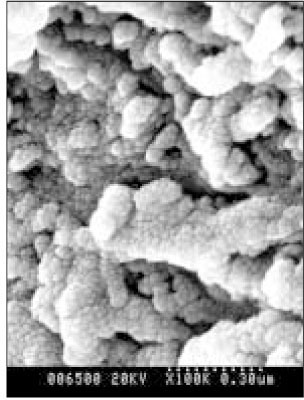
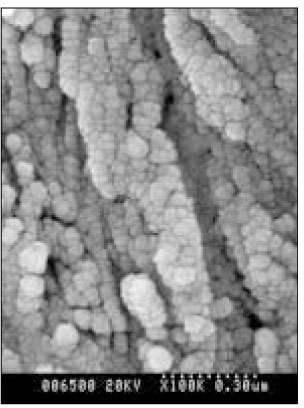
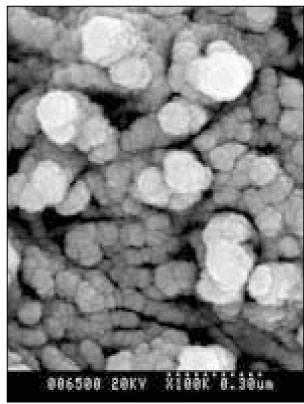
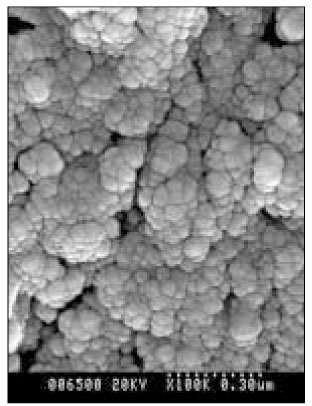
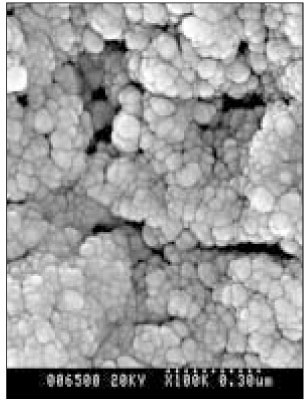
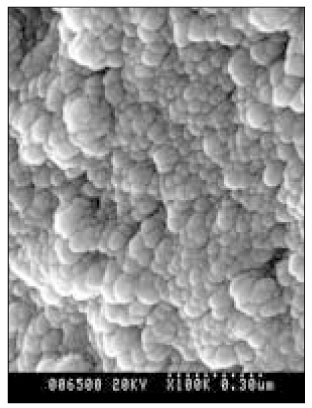
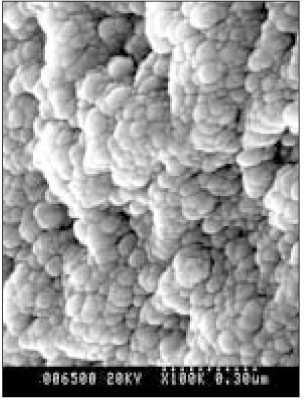
- Dental caries is the most common disease in the oral cavity. However, the mechanism and treatment of dental caries is not completely understood since many complex factors are involved. Especially the effect of pH on remineralization of early stage of dental caries is still controversial. In this study, dental caries in dentin was induced by using lactic acidulated buffering solutions and the loss of inorganic substance was measured. Also decalcified specimens were remineralized by three groups of solution with different pH (group of pH 4.3, 5.0, and 5.5). Then, the amount and the area of inorganic substance precipitation was quantitatively analyzed with microradiograph. Also a qualitative comparison of the normal phase, the demineralized phase, and the remineralized phase of hydroxyapatite crystal was made under SEM. The results were as follows; 1. In microradiograghic analysis, as the pH increased, the amount of remineralization in decalcified dentin tended to increase significantly. As the pH decreaced, deeper decalcification, however, occurred along with remineralization. The group of pH 5.5 had a tendency to be remineralized without demineralization (p < 0.05). 2. In SEM view, the remineralization in dentine caries occurred from the hydroxyapatite crystal surface surrounding the mesh of organic matrix, and eventually filled up the demineralized area. 3. 5 days after remineralization, hydroxyapatite crystal grew bigger with deposition of inorganic substance in pH 4.3 and 5.0 group, and the crystal in the remineralized area appeared to return to normal. After 10 days, the crystals in group of pH 4.3 and 5.0, which grew bigger after 5 days of remineralization, turned back to their normal size, but in group of pH 5.5, some crystals were found to double their size. In according to the results of this experiment, the decalcifying and remineralizing process of dentine is neither simple nor independent, but a dynamic process in which decalcification and remineralization occur simultaneously. The remineralization process occurred from the hydroxyapatite crystal surface.
Figure
Cited by 1 articles
-
The remineralizing features of pH 5.5 solutions of different degree of saturations on artificially demineralized enamel
Young-Jun Kwak, Eui-seoug Kim, Sung-Ho Park, Hyung Kyu Gong, Yoon Lee, Chan-Young Lee
J Korean Acad Conserv Dent. 2008;33(5):481-492. doi: 10.5395/JKACD.2008.33.5.481.
Reference
-
1. Arends J, Jongebloed W, Ogaard B, Rolla G. SEM and microradiographic investigation of initial enamel caries. Scand J Dent Res. 1987. 95(3):193–201.
Article2. Bowes DN, Dunn EJ. A simple vaccum cassette for microradiography. Stain Technol. 1975. 50:355–357.3. Chow LC, Takaki S. Remineralization of root lesions with concentrated Ca and P solutions. Dent Mater J. 1995. 14(1):31–36.4. Christensen GJ. A new challenge-root caries in mature people. J Am Dent Assoc. 1996. 127:379–380.
Article5. Featherstone JDB. Comparison of artificial caries like lesions by quantitative microradiography and microhardness profile. Caries Res. 1983. 17:385–391.
Article6. Holmen L, Thystrup A, Featherstone JDB, Fredebo L, Shariati M. A scanning electron microscopic study of surface changes during development of artificial caries. Caries Res. 1985. 19:11–21.
Article7. Inaba D, Ruben J, Arends J. A computer-assisted videodensitometric method to visualize mineral distributions in in vitro and in vivo formed root caries lesion. Eur J Oral Sci. 1997. 105:74–80.
Article8. Katz RV. Root caries: Clinical implication of the current epidemiologic data. Northwest Dent. 1981. 60(6):306–310.9. Kawasaki K, Ruben J, Takagi O. Relationship between mineral distributions in dentin lesions and subsequent remineralization in vitro. Caries Res. 2000. 34:395. 403.
Article10. Koch G, Petersson LG, Kling E. Effect of 250 and 1000ppm fluoridr dentifrice on caries. A three-year clinical study. Swed Dent J. 1982. 6(6):233–238.11. Margolis HC, Moreno EC, Murphy BJ. Effect of low levels of fluoride in solution on enamel demineralization. J Dent Res. 1986. 65:23–29.
Article12. Moreno EC, Margolis HC. Composition of human plaque fluid. J Dent Res. 1988. 67(9):1181–1189.
Article13. Mukai Y, Ten cate JM. Remineralization of advanced root dentin lesions in vitro. Caries Res. 2002. 36:275–280.
Article14. Rooij JF, Nancollas GH. The formation and remineralization of artificial white spot lesion. J Dent Res. 1984. 63(6):864–867.15. Silverstone LM, Hicks MJ, Featherstone MJ. Dynamic facters affecting lesion initiation and progression in human dental enamel. Quintessence Int. 1988. 19:773–785.16. Cate JM, Arends J. Remineralization of artificial enamel iesions in vitro. Caries Res. 1977. 11:277–284.17. Theuns HM, van Dijk JWE, Driessens FCM, Groeneveld A. Effect of the pH of buffer solutions on artificial carious lesion formation in human tooth enamel. Caries Res. 1984. 18:7–11.
Article19. Wefel JS, Heilman JR. Natural root caries : A histologic and microradiographic evaluation. J Oral Path. 1985. 14:615–623.20. White DJ, Chen WC, Nancollas GH. Kinetic and physical aspects of enamel remineralization. Caries Res. 1988. 22:11–19.
Article21. Kum KY, Lee CY. The effect of four kinds of acid and concentration on the formation of artificial carious lesion in human tooth enamel. J Korean Acad Conserv Dent. 1996. 21:470–488.22. Park SH, Lee CY, Lee CS. The effect of acid concentration and ph of lactate buffer solution on the progress of artificial caries lesion in human tooth enamel. J Korean Acad Conserv Dent. 1993. 18:277–290.23. Park JW, Hur B, Lee CY. The effects of the degree of saturation of acidulated buffer solutions in enamel and dentin remineralization and afm observation of hydroxyapatite crystals. J Korean Acad Conserv Dent. 2000. 25:459–473.24. Lee CY. Artificial caries formation in acid buffering solution. Yonsei Dent J. 1992. 7:34–41.25. Han WS, Kum KY, Lee CY. The influence of fluoride on remineralization of artificial dental caries. J Korean Acad Conserv Dent. 1996. 21:161–173.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo quantitative analysis of remineralization effect of remineralization solution "R" of incipient enamel dental caries
- The effect of lactic acid concentration and ph of lactic acid buffer solutions on enamel remineralization
- The change of the configuration of hydroxyapatite crystals in enamel by changes of pH and degree of saturation of lactic acid buffer solution
- The effects of the fluoride concentration of acidulated buffer solutions on dentine remineralization
- Effect of casein phosphopeptide-amorphous calcium phosphate on fluoride release and micro-shear bond strength of resin-modified glass ionomer cement in caries-affected dentin