J Korean Acad Conserv Dent.
2004 Jul;29(4):370-377. 10.5395/JKACD.2004.29.4.370.
Tissue engineering of dental pulp on type I collagen
- Affiliations
-
- 1Department of Dental Hygiene, Wonkwang Health Science College, Korea.
- 2Department of Dental Technology, Shingu College, Korea.
- 3Department of Conservative Dentistry, College of Dentistry, Kyung-Hee University, Korea. shpark94@khu.ac.kr
- KMID: 2175631
- DOI: http://doi.org/10.5395/JKACD.2004.29.4.370
Abstract
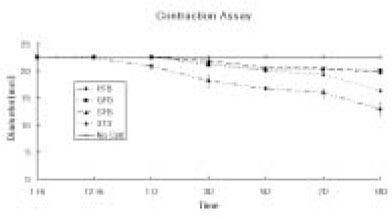
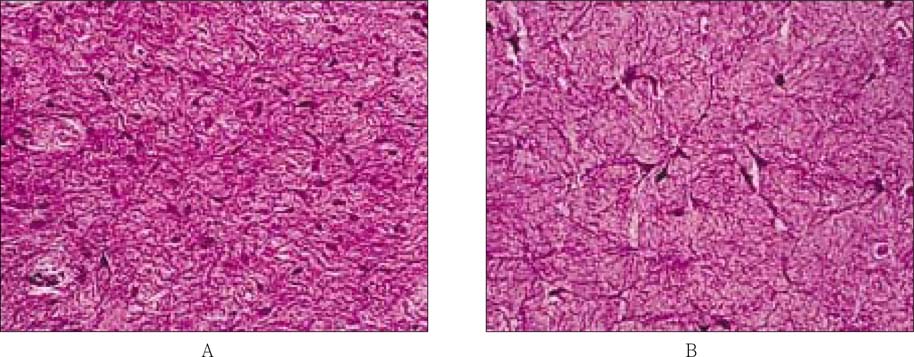
- The purpose of this study was to regenerate human dental pulp tissues similar to native pulp tissues. Using the mixture of type I collagen solution, primary cells collected from the different tissues (pulp, gingiva, and skin) and NIH 3T3 (1 x 10(5) cells/ml/well) were cultured at 12-well plate at 37degrees C for 14 days. Standardized photographs were taken with digital camera during 14 days and the diameter of the contracted collagen gel matrix was measured and statistically analyzed with student t-test. As one of the pulp tissue engineering, normal human dental pulp tissue and collagen gel matrix cultured with dental pulp cells for 14 days were fixed and stained with Hematoxyline & Eosin. According to this study, the results were as follows: 1. The contraction of collagen gel matrix cultured with pulp cells for 14 days was significantly higher than other fibroblasts (gingiva, skin) (p < 0.05). 2. The diameter of collagen gel matrix cultured with pulp cells was reduced to 70.4% after 7 days, and 57.1% after 14 days. 3. The collagen gel without any cells did not contract, whereas the collagen gel cultured with gingiva and skin showed mild contraction after 14 days (88.1% and 87.6% respectively). 4. The contraction of the collagen gel cultured with NIH 3T3 cells after 14 days was higher than those cultured with gingival and skin fibroblasts, but it was not statistically significant (72.1%, p > 0.05). 5. The collagen gel matrix cultured with pulp cells for 14 days showed similar shape with native pulp tissue without blood vessels. This approach may provide a means of engineering a variety of other oral tissue as well and these cell behaviors may provide information needed to establish pulp tissue engineering protocols.
Keyword
MeSH Terms
Figure
Reference
-
1. Dard M, Sewing A, Meyer J, Verrier S, Roessler S, Scharnweber D. Tools for tissue engineering of mineralized oral structures. Clin Oral Investig. 2000. 4:126–129.
Article2. Mooney DJ, Powell C, Piana J, Rutherford B. Engineering dental pulp-like tissue in Vitro. Biotechnol Prog. 1996. 12:865–868.3. Grinnell . Fibroblast biology in three-dimensional collagen matrix. Trends in Cell Biology. 2003. 13:264–269.
Article4. Mikos AG, Mcintire LV. Frontiers in tissue engineering. 1998. Pergamon. Houston. TX:5. Hargreaves KM, Goodis HE. Seltzer and Bender's Dental Pulp. 2002. Chicago. IL: Quintessence Publishing Co. Inc.6. Putnam AJ, Mooney DJ. Tissue engineering using synthetic extracellular matrices. Nature Med. 1996. 2:824–826.
Article7. Buurma B, Gu K, Rutherford R. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. Eur J Oral Sci. 1999. 107:282–289.
Article8. Bohl KS, Shon J, Rutherford B, Mooney DJ. Role of synthetic extracellular matrix in development of engineered dental pulp. J Biomater Sci Polym Ed. 1998. 9:749–764.
Article9. Vernon RB, Sage EH. Contraction of fibrillar type I collagen by endothelial cells: A study in vitro. J Cell Biochem. 1996. 60:185–197.
Article10. Zhu YK, Umino T, Liu XD, Wang HJ, Romberger DJ, Spurzem JR, Rennard SI. Contraction of fibroblast-containing collagen gels: initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell Dev Biol Anim. 2001. 37:10–16.
Article11. Park SH. Culturing the human dental pulp cells in the collagen matrix and on the ground tooth surface. J Korean Acad Conserv Dent. 2003. 28:419–424.
Article12. Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972. 54:626–637.
Article13. Brock DP, Marty-Raix R, Spector M. a-Smooth-muscle actin in and contraction of porcine dental pulp cells. J Dent Res. 2002. 81:203–208.
Article14. Schor SL. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980. 41:159–175.15. Rhudy RW, Mcpherson JM. Influence of the extracellular matrix on the proliferative response of human skin fibroblasts to serum and purified platelet-derived growth factor. J Cell Physiol. 1988. 137:185–191.
Article16. Burg KJL, Holder WD, Culberson CR, Beiler RJ, Greeene KG, Loebsack AB, Roland WD, Eiselt P, Mooney DJ, Halberstadt CR. Comparative study of seeding methods for three-dimensional polymeric scaffolds. J Biomed Mater Res. 2000. 51:642–649.
Article17. Alsberg E, Anderson KW, Alberiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001. 80:2025–2029.
Article18. Young CS, Terada S, Vacanti JP, Honda M, Barrtlett JD, Yelick PC. Tissue engineering of complex tooth stuructures on biodegradable polymer scaffolds. J Dent Res. 2002. 81:695–700.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in Research on Stem Cell-Based Pulp Regeneration
- Culturing the Human Dental pulp cells in the Collagen Matrix and on the ground tooth surface
- Co-culture of Human Dental Pulp Stem Cells and Endothelial Cells Using Porous Biopolymer Microcarriers: A Feasibility Study for Bone Tissue Engineering
- Pulp tissue regeneration and root formation of permanent teeth with pulpal/periapical deseases
- Effects of Type I Collagen Concentration in Hydrogel on the Growth and Phenotypic Expression of Rat Chondrocytes