Diabetes Metab J.
2012 Oct;36(5):328-335. 10.4093/dmj.2012.36.5.328.
Transcriptional Regulation of Pyruvate Dehydrogenase Kinase
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea. leei@knu.ac.kr
- 2Department of Fundamental Medical & Pharmaceutical Sciences, Catholic University of Daegu, Daegu, Korea.
- KMID: 2174390
- DOI: http://doi.org/10.4093/dmj.2012.36.5.328
Abstract
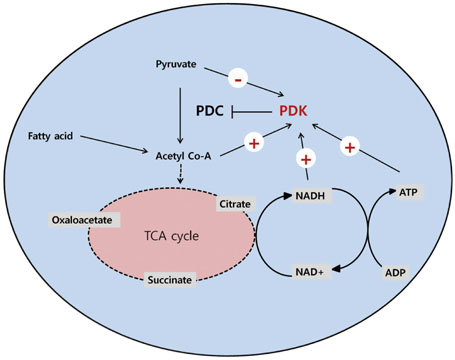
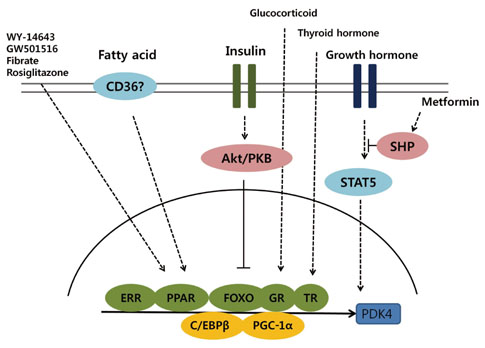
- The pyruvate dehydrogenase complex (PDC) activity is crucial to maintains blood glucose and ATP levels, which largely depends on the phosphorylation status by pyruvate dehydrogenase kinase (PDK) isoenzymes. Although it has been reported that PDC is phosphorylated and inactivated by PDK2 and PDK4 in metabolically active tissues including liver, skeletal muscle, heart, and kidney during starvation and diabetes, the precise mechanisms by which expression of PDK2 and PDK4 are transcriptionally regulated still remains unclear. Insulin represses the expression of PDK2 and PDK4 via phosphorylation of FOXO through PI3K/Akt signaling pathway. Several nuclear hormone receptors activated due to fasting or increased fat supply, including peroxisome proliferator-activated receptors, glucocorticoid receptors, estrogen-related receptors, and thyroid hormone receptors, also participate in the up-regulation of PDK2 and PDK4; however, the endogenous ligands that bind those nuclear receptors have not been identified. It has been recently suggested that growth hormone, adiponectin, epinephrine, and rosiglitazone also control the expression of PDK4 in tissue-specific manners. In this review, we discuss several factors involved in the expressional regulation of PDK2 and PDK4, and introduce current studies aimed at providing a better understanding of the molecular mechanisms that underlie the development of metabolic diseases such as diabetes.
Keyword
MeSH Terms
-
Adenosine Triphosphate
Adiponectin
Blood Glucose
Epinephrine
Fasting
Growth Hormone
Heart
Insulin
Insulin Resistance
Isoenzymes
Kidney
Ligands
Liver
Metabolic Diseases
Muscle, Skeletal
Oxidoreductases
Peroxisome Proliferator-Activated Receptors
Phosphorylation
Phosphotransferases
Protein-Serine-Threonine Kinases
Pyruvate Dehydrogenase Complex
Pyruvic Acid
Receptors, Cytoplasmic and Nuclear
Receptors, Glucocorticoid
Receptors, Thyroid Hormone
Starvation
Thiazolidinediones
Up-Regulation
Adenosine Triphosphate
Adiponectin
Blood Glucose
Epinephrine
Growth Hormone
Insulin
Isoenzymes
Ligands
Oxidoreductases
Peroxisome Proliferator-Activated Receptors
Phosphotransferases
Protein-Serine-Threonine Kinases
Pyruvate Dehydrogenase Complex
Pyruvic Acid
Receptors, Cytoplasmic and Nuclear
Receptors, Glucocorticoid
Receptors, Thyroid Hormone
Thiazolidinediones
Figure
Cited by 1 articles
-
Mechanisms of Vascular Calcification: The Pivotal Role of Pyruvate Dehydrogenase Kinase 4
Jaechan Leem, In-Kyu Lee
Endocrinol Metab. 2016;31(1):52-61. doi: 10.3803/EnM.2016.31.1.52.
Reference
-
1. Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem. 2006. 112:139–149.2. Wu P, Blair PV, Sato J, Jaskiewicz J, Popov KM, Harris RA. Starvation increases the amount of pyruvate dehydrogenase kinase in several mammalian tissues. Arch Biochem Biophys. 2000. 381:1–7.3. Holness MJ, Bulmer K, Smith ND, Sugden MC. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem J. 2003. 369(Pt 3):687–695.4. Wu P, Sato J, Zhao Y, Jaskiewicz J, Popov KM, Harris RA. Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J. 1998. 329(Pt 1):197–201.5. Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999. 48:1593–1599.6. Stavinoha MA, Rayspellicy JW, Hart-Sailors ML, Mersmann HJ, Bray MS, Young ME. Diurnal variations in the responsiveness of cardiac and skeletal muscle to fatty acids. Am J Physiol Endocrinol Metab. 2004. 287:E878–E887.7. Rosa G, Di Rocco P, Manco M, Greco AV, Castagneto M, Vidal H, Mingrone G. Reduced PDK4 expression associates with increased insulin sensitivity in postobese patients. Obes Res. 2003. 11:176–182.8. Kim YI, Lee FN, Choi WS, Lee S, Youn JH. Insulin regulation of skeletal muscle PDK4 mRNA expression is impaired in acute insulin-resistant states. Diabetes. 2006. 55:2311–2317.9. Kwon HS, Huang B, Unterman TG, Harris RA. Protein kinase B-alpha inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes. 2004. 53:899–910.10. Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J. 2003. 375(Pt 2):365–371.11. Nahle Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. J Biol Chem. 2008. 283:14317–14326.12. Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin. Diabetes. 2002. 51:276–283.13. Houten SM, Chegary M, Te Brinke H, Wijnen WJ, Glatz JF, Luiken JJ, Wijburg FA, Wanders RJ. Pyruvate dehydrogenase kinase 4 expression is synergistically induced by AMP-activated protein kinase and fatty acids. Cell Mol Life Sci. 2009. 66:1283–1294.14. Zungu M, Young ME, Stanley WC, Essop MF. Chronic treatment with the peroxisome proliferator-activated receptor alpha agonist Wy-14,643 attenuates myocardial respiratory capacity and contractile function. Mol Cell Biochem. 2009. 330:55–62.15. Wan J, Jiang L, Lu Q, Ke L, Li X, Tong N. Activation of PPARdelta up-regulates fatty acid oxidation and energy uncoupling genes of mitochondria and reduces palmitate-induced apoptosis in pancreatic beta-cells. Biochem Biophys Res Commun. 2010. 391:1567–1572.16. Zhao Y, Okuyama M, Hashimoto H, Tagawa Y, Jomori T, Yang B. Bezafibrate induces myotoxicity in human rhabdomyosarcoma cells via peroxisome proliferator-activated receptor alpha signaling. Toxicol In Vitro. 2010. 24:154–159.17. Caton PW, Holness MJ, Bishop-Bailey D, Sugden MC. PPARalpha-LXR as a novel metabolostatic signalling axis in skeletal muscle that acts to optimize substrate selection in response to nutrient status. Biochem J. 2011. 437:521–530.18. Degenhardt T, Saramaki A, Malinen M, Rieck M, Vaisanen S, Huotari A, Herzig KH, Muller R, Carlberg C. Three members of the human pyruvate dehydrogenase kinase gene family are direct targets of the peroxisome proliferator-activated receptor beta/delta. J Mol Biol. 2007. 372:341–355.19. Cadoudal T, Distel E, Durant S, Fouque F, Blouin JM, Collinet M, Bortoli S, Forest C, Benelli C. Pyruvate dehydrogenase kinase 4: regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes. 2008. 57:2272–2279.20. Augustus A, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J Biol Chem. 2004. 279:25050–25057.21. Brown JD, Oligino E, Rader DJ, Saghatelian A, Plutzky J. VLDL hydrolysis by hepatic lipase regulates PPARdelta transcriptional responses. PLoS One. 2011. 6:e21209.22. Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D. Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: potential role of intramuscular long-chain acyl-coenzyme A. J Clin Endocrinol Metab. 2007. 92:3967–3972.23. Lee FN, Zhang L, Zheng D, Choi WS, Youn JH. Insulin suppresses PDK-4 expression in skeletal muscle independently of plasma FFA. Am J Physiol Endocrinol Metab. 2004. 287:E69–E74.24. Qi D, Pulinilkunnil T, An D, Ghosh S, Abrahani A, Pospisilik JA, Brownsey R, Wambolt R, Allard M, Rodrigues B. Single-dose dexamethasone induces whole-body insulin resistance and alters both cardiac fatty acid and carbohydrate metabolism. Diabetes. 2004. 53:1790–1797.25. Araki M, Motojima K. Identification of ERRalpha as a specific partner of PGC-1alpha for the activation of PDK4 gene expression in muscle. FEBS J. 2006. 273:1669–1680.26. Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA. Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem. 2006. 281:39897–39906.27. Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Stimulation of carnitine acylcarnitine translocase activity in heart mitochondria from hyperthyroid rats. FEBS Lett. 1996. 397:260–262.28. Priestman DA, Donald E, Holness MJ, Sugden MC. Different mechanisms underlie the long-term regulation of pyruvate dehydrogenase kinase (PDHK) by tri-iodothyronine in heart and liver. FEBS Lett. 1997. 419:55–57.29. Orfali KA, Fryer LG, Holness MJ, Sugden MC. Interactive effects of insulin and triiodothyronine on pyruvate dehydrogenase kinase activity in cardiac myocytes. J Mol Cell Cardiol. 1995. 27:901–908.30. Sugden MC, Fryer LG, Priestman DA, Orfali KA, Holness MJ. Increased hepatic pyruvate dehydrogenase kinase activity in fed hyperthyroid rats: studies in vivo and with cultured hepatocytes. Mol Cell Endocrinol. 1996. 119:219–224.31. Sugden MC, Langdown ML, Harris RA, Holness MJ. Expression and regulation of pyruvate dehydrogenase kinase isoforms in the developing rat heart and in adulthood: role of thyroid hormone status and lipid supply. Biochem J. 2000. 352(Pt 3):731–738.32. Hyyti OM, Ning XH, Buroker NE, Ge M, Portman MA. Thyroid hormone controls myocardial substrate metabolism through nuclear receptor-mediated and rapid posttranscriptional mechanisms. Am J Physiol Endocrinol Metab. 2006. 290:E372–E379.33. Attia RR, Connnaughton S, Boone LR, Wang F, Elam MB, Ness GC, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by thyroid hormone: role of the peroxisome proliferator-activated receptor gamma coactivator (PGC-1 alpha). J Biol Chem. 2010. 285:2375–2385.34. Attia RR, Sharma P, Janssen RC, Friedman JE, Deng X, Lee JS, Elam MB, Cook GA, Park EA. Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by CCAAT/enhancer-binding protein beta (C/EBPbeta). J Biol Chem. 2011. 286:23799–23807.35. Raboudi N, Arem R, Jones RH, Chap Z, Pena J, Chou J, Field JB. Fasting and postabsorptive hepatic glucose and insulin metabolism in hyperthyroidism. Am J Physiol. 1989. 256(1 Pt 1):E159–E166.36. Zhang Y, Ma K, Song S, Elam MB, Cook GA, Park EA. Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha). J Biol Chem. 2004. 279:53963–53971.37. Aylward JH, Bornstein J, Gould MK, Hall S. Inhibition of muscle pyruvate dehydrogenase by a polypeptide from growth hormone. Biochem Biophys Res Commun. 1974. 59:57–61.38. Clot JP, Benelli C, de Galle B, Postel-Vinay MC, Durand D, Desbuquois B. Effects of growth hormone on pyruvate dehydrogenase activity in intact rat liver and in isolated hepatocytes: comparison with insulin. Metabolism. 1988. 37:1101–1106.39. Clot JP, Benelli C, Fouque F, Bernard R, Durand D, Postel-Vinay MC. Pyruvate dehydrogenase activity is stimulated by growth hormone (GH) in human mononuclear cells: a new tool to measure GH responsiveness in man. J Clin Endocrinol Metab. 1992. 74:1258–1262.40. Seiva FR, Berbert CM, Souza GA, Rocha KK, Ebaid GM, Burneiko RC, Novelli EL. Energy expenditure, lipid profile, oxidative stress, and cardiac energy metabolism after growth hormone treatment in obese young rats. Horm Metab Res. 2010. 42:496–501.41. White UA, Coulter AA, Miles TK, Stephens JM. The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes. 2007. 56:1623–1629.42. Kim YD, Kim YH, Tadi S, Yu JH, Yim YH, Jeoung NH, Shong M, Hennighausen L, Harris RA, Lee IK, Lee CH, Choi HS. Metformin inhibits growth hormone-mediated hepatic pyruvate dehydrogenase kinase 4 gene expression through induction of orphan nuclear receptor small heterodimer partner. Diabetes. 2012. 61:2484–2494.43. McAinch AJ, Cameron-Smith D. Adiponectin decreases pyruvate dehydrogenase kinase 4 gene expression in obese- and diabetic-derived myotubes. Diabetes Obes Metab. 2009. 11:721–728.44. Nye C, Kim J, Kalhan SC, Hanson RW. Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab. 2008. 19:356–361.45. Wan Z, Thrush AB, Legare M, Frier BC, Sutherland LN, Williams DB, Wright DC. Epinephrine-mediated regulation of PDK4 mRNA in rat adipose tissue. Am J Physiol Cell Physiol. 2010. 299:C1162–C1170.46. Wan Z, Frier BC, Williams DB, Wright DC. Epinephrine induces PDK4 mRNA expression in adipose tissue from obese, insulin resistant rats. Obesity (Silver Spring). 2012. 20:453–456.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Pyruvate Dehydrogenase Kinase in Diabetes and Obesity
- Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases
- Pyruvate Dehydrogenase Kinases: Therapeutic Targets for Diabetes and Cancers
- Role of Pyruvate Dehydrogenase Kinase 4 in Regulation of Blood Glucose Levels
- Red Blood Cell Enzymopathies Causing Hereditary Hemolytic Anemia