Intest Res.
2014 Jan;12(1):34-41. 10.5217/ir.2014.12.1.34.
Parthenolide Sensitizes Human Colorectal Cancer Cells to Tumor Necrosis Factor-related Apoptosis-inducing Ligand through Mitochondrial and Caspase Dependent Pathway
- Affiliations
-
- 1Department of Internal Medicine, Biomedical Research Institute, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea. clickm@jbnu.ac.kr
- 2Research Institute of Clinical Medicine, Biomedical Research Institute, Chonbuk National University Hospital, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2174335
- DOI: http://doi.org/10.5217/ir.2014.12.1.34
Abstract
- BACKGROUND/AIMS
Combination therapy utilizing tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) in conjunction with other anticancer agents, is a promising strategy to overcome TRAIL resistance in malignant cells. Recently, parthenolide (PT) has proved to be a promising anticancer agent, and several studies have explored its use in combination therapy. Here, we investigated the molecular mechanisms by which PT sensitizes colorectal cancer (CRC) cells to TRAIL-induced apoptosis.
METHODS
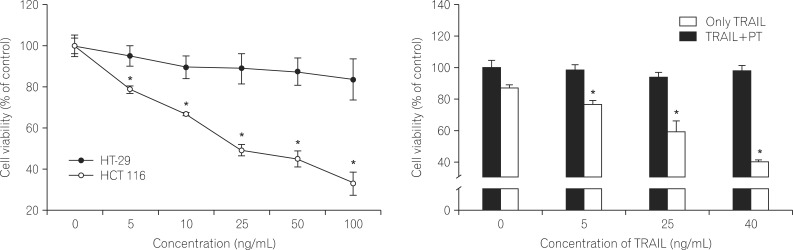
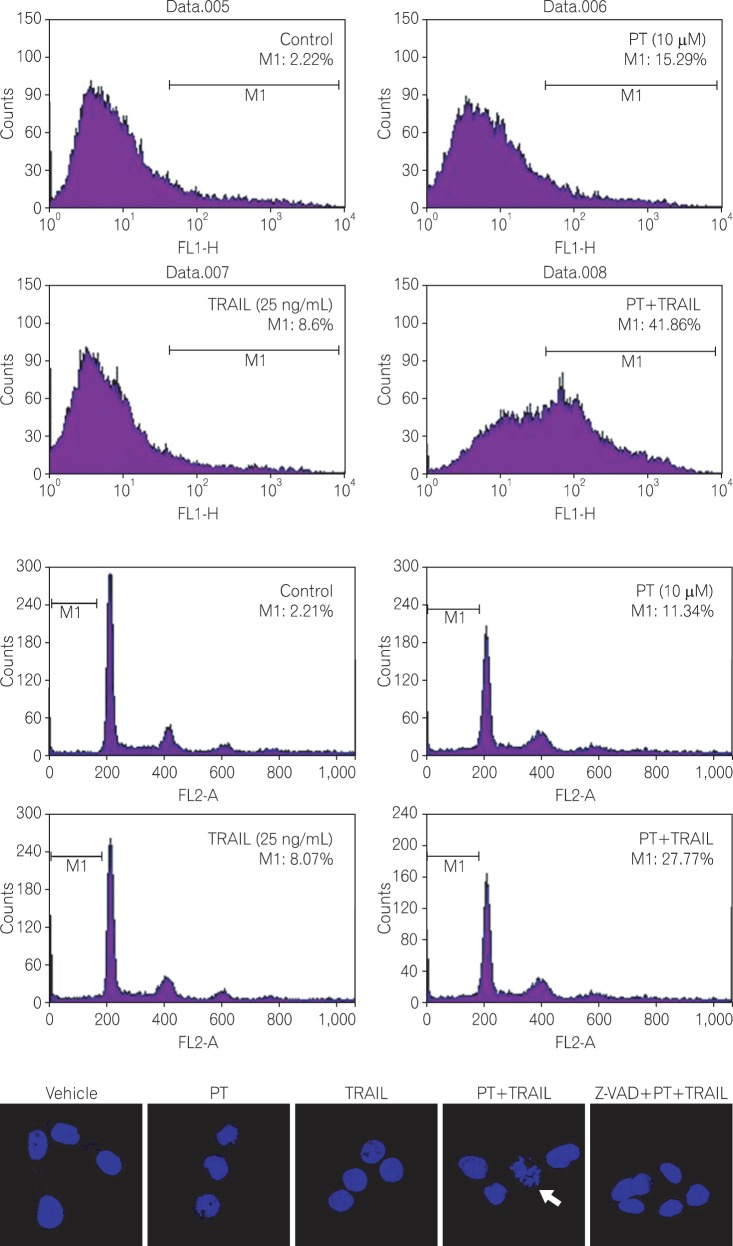
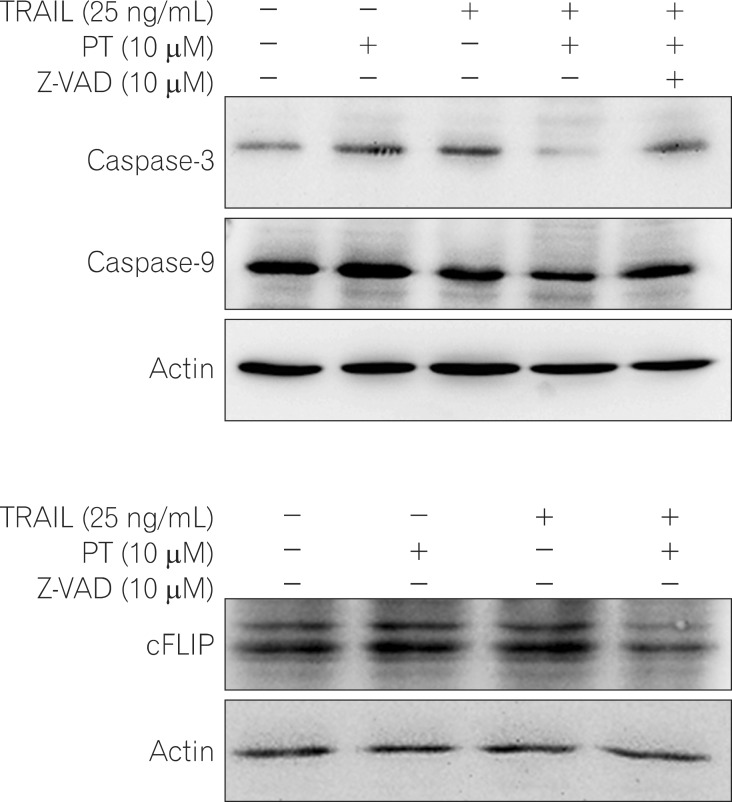
HT-29 cells (TRAIL-resistant) were treated with PT and/or TRAIL for 24 hours. The inhibitory effect on proliferation was detected using the 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Annexin V staining, cell cycle analysis, and Hoechst 33258 staining were used to assess apoptotic cell death. Activation of an apoptotic pathway was confirmed by Western blot.
RESULTS
Treatment with TRAIL alone inhibited the proliferation of HCT 116 cells in a dose-dependent manner, whereas proliferation was not affected in HT-29 cells. Combination PT and TRAIL treatment significantly inhibited cell growth and induced apoptosis of HT-29 cells. We observed that the synergistic effect was associated with misregulation of B-cell lymphoma 2 (Bcl-2) family members, release of cytochrome C to the cytosol, activation of caspases, and increased levels of p53.
CONCLUSION
Combination therapy using PT and TRAIL might offer an effetive strategy to overcome TRAIL resistance in certain CRC cells.
Keyword
MeSH Terms
-
Annexin A5
Antineoplastic Agents
Apoptosis
Bisbenzimidazole
Blotting, Western
Caspases
Cell Cycle
Cell Death
Colorectal Neoplasms*
Cytochromes c
Cytosol
HCT116 Cells
HT29 Cells
Humans*
Lymphoma, B-Cell
Necrosis*
Tumor Necrosis Factor-alpha
Annexin A5
Antineoplastic Agents
Bisbenzimidazole
Caspases
Cytochromes c
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Balsalazide Potentiates Parthenolide-Mediated Inhibition of Nuclear Factor-κB Signaling in HCT116 Human Colorectal Cancer Cells
Hyun-Young Kim, Se-Lim Kim, Young-Ran Park, Yu-Chuan Liu, Seung Young Seo, Seong Hun Kim, In Hee Kim, Seung Ok Lee, Soo Teik Lee, Sang Wook Kim
Intest Res. 2015;13(3):233-241. doi: 10.5217/ir.2015.13.3.233.
Reference
-
1. Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010; 29:4752–4765. PMID: 20531300.
Article2. Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008; 8:782–798. PMID: 18813321.
Article3. Shi YG. Mechanisms of caspase activation and inhibition during apoptosis. Molecular Cell. 2002; 9:459–470. PMID: 11931755.
Article4. Koschny R, Walczak H, Ganten TM. The promise of TRAIL - potential and risks of a novel anticancer therapy. J Mol Med (Berl). 2007; 85:923–935. PMID: 17437073.5. Jin Z, McDonald ER, Dicker DT, El-Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem. 2004; 279:35829–35839. PMID: 15155747.
Article6. Kim HS, Lee JW, Soung YH, et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology. 2003; 125:708–715. PMID: 12949717.
Article7. Hernandez A, Wang QD, Schwartz SA, Evers BM. Sensitization of human colon cancer cells to TRAIL-mediated apoptosis. J Gastrointest Surg. 2001; 5:56–65. PMID: 11309649.
Article8. Burns TF, El-Deiry WS. Identification of inhibitors of TRAIL-induced death (ITIDs) in the TRAIL-sensitive colon carcinoma cell line SW480 using a genetic approach. J Biol Chem. 2001; 276:37879–37886. PMID: 11486001.
Article9. Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004; 64:3006–3008. PMID: 15126334.
Article10. Carlisi D, D'Anneo A, Angileri L, et al. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol. 2011; 226:1632–1641. PMID: 21413021.
Article11. Seo OW, Kim JH, Lee KS, et al. Kurarinone promotes TRAIL-induced apoptosis by inhibiting NF-kappaB-dependent cFLIP expression in HeLa cells. Exp Mol Med. 2012; 44:653–664. PMID: 22932446.
Article12. Kauntz H, Bousserouel S, Gosse F, Raul F. The flavonolignan silibinin potentiates TRAIL-induced apoptosis in human colon adenocarcinoma and in derived TRAIL-resistant metastatic cells. Apoptosis. 2012; 17:797–809. PMID: 22555452.
Article13. Knight DW. Feverfew: chemistry and biological activity. Nat Prod Rep. 1995; 12:271–276. PMID: 7792073.
Article14. Sohma I, Fujiwara Y, Sugita Y, et al. Parthenolide, an NF-kappaB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics. 2011; 8:39–47. PMID: 21289336.15. Zhao LJ, Xu YH, Li Y. Effect of parthenolide on proliferation and apoptosis in gastric cancer cell line SGC7901. J Dig Dis. 2009; 10:172–180. PMID: 19659784.
Article16. Park JH, Liu L, Kim IH, Kim JH, You KR, Kim DG. Identification of the genes involved in enhanced fenretinide-induced apoptosis by parthenolide in human hepatoma cells. Cancer Res. 2005; 65:2804–2814. PMID: 15805281.
Article17. Zanotto-Filho A, Braganhol E, Schroder R, et al. NFkappaB inhibitors induce cell death in glioblastomas. Biochem Pharmacol. 2011; 81:412–424. PMID: 21040711.18. Dai Y, Guzman ML, Chen S, et al. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol. 2010; 151:70–83. PMID: 20701602.
Article19. Sun Y, St Clair DK, Xu Y, Crooks PA, St Clair WH. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res. 2010; 70:2880–2890. PMID: 20233868.
Article20. Zhang S, Ong CN, Shen HM. Involvement of proapoptotic Bcl-2 family members in parthenolide-induced mitochondrial dysfunction and apoptosis. Cancer Lett. 2004; 211:175–188. PMID: 15219941.
Article21. Kim SL, Trang KT, Kim SH, et al. Parthenolide suppresses tumor growth in a xenograft model of colorectal cancer cells by inducing mitochondrial dysfunction and apoptosis. Int J Oncol. 2012; 41:1547–1553. PMID: 22895542.
Article22. Fang LJ, Shao XT, Wang S, Lu GH, Xu T, Zhou JY. Sesquiterpene lactone parthenolide markedly enhances sensitivity of human A549 cells to low-dose oxaliplatin via inhibition of NF-kappaB activation and induction of apoptosis. Planta Med. 2010; 76:258–264. PMID: 19774508.
Article23. Gao ZW, Zhang DL, Guo CB. Paclitaxel efficacy is increased by parthenolide via nuclear factor-kappaB pathways in in vitro and in vivo human non-small cell lung cancer models. Curr Cancer Drug Targets. 2010; 10:705–715. PMID: 20578985.24. Yun BR, Lee MJ, Kim JH, Kim IH, Yu GR, Kim DG. Enhancement of parthenolide-induced apoptosis by a PKC-alpha inhibition through heme oxygenase-1 blockage in cholangiocarcinoma cells. Exp Mol Med. 2010; 42:787–797. PMID: 20938215.
Article25. Lee CS, Kim YJ, Lee SA, Myung SC, Kim W. Combined effect of Hsp90 inhibitor geldanamycin and parthenolide via reactive oxygen species-mediated apoptotic process on epithelial ovarian cancer cells. Basic Clin Pharmacol Toxicol. 2012; 111:173–181. PMID: 22433057.
Article26. Yip-Schneider MT, Nakshatri H, Sweeney CJ, Marshall MS, Wiebke EA, Schmidt CM. Parthenolide and sulindac cooperate to mediate growth suppression and inhibit the nuclear factor-kappa B pathway in pancreatic carcinoma cells. Mol Cancer Ther. 2005; 4:587–594. PMID: 15827332.
Article27. Kim SL, Kim SH, Trang KT, et al. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer. Cancer Lett. 2013; 335:479–486. PMID: 23507557.
Article28. Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004; 23:7330–7344. PMID: 15286701.
Article29. Fulda S, Debatin KM. Death receptor signaling in cancer therapy. Curr Med Chem Anticancer Agents. 2003; 3:253–262. PMID: 12769771.
Article30. Poukkula M, Kaunisto A, Hietakangas V, et al. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem. 2005; 280:27345–27355. PMID: 15886205.
Article31. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990; 61:759–767. PMID: 2188735.
Article32. Lee SC, Cheong HJ, Kim SJ, et al. Low-Dose Combinations of LBH589 and TRAIL Can Overcome TRAIL-resistance in Colon Cancer Cell Lines. Anticancer Res. 2011; 31:3385–3394. PMID: 21965751.33. Park MH, Jo M, Won D, Song HS, Song MJ, Hong JT. Snake venom toxin from Vipera lebetina turanica sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins. Apoptosis. 2012; 17:1316–1326. PMID: 23007278.
Article34. Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994; 269:30761–30764. PMID: 7983002.
Article36. Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996; 88:386–401. PMID: 8695785.
Article37. Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998; 252:1–15. PMID: 9523706.
Article38. Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997; 387:773–776. PMID: 9194558.
Article39. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995; 80:293–299. PMID: 7834749.
Article40. Sionov RV, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene. 1999; 18:6145–6157. PMID: 10557106.
Article41. Ullenhag GJ, Mukherjee A, Watson NF, Al-Attar AH, Scholefield JH, Durrant LG. Overexpression of FLIPL is an independent marker of poor prognosis in colorectal cancer patients. Clin Cancer Res. 2007; 13:5070–5075. PMID: 17785559.
Article42. Wang X, Chen W, Zeng W, et al. Akt-mediated eminent expression of c-FLIP and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity to lung cancer cells. Mol Cancer Ther. 2008; 7:1156–1163. PMID: 18483303.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytotoxic Effects of Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL)and its Molecular Mechanism in Human Gastric Cancer Cells
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons
- The Synergistic Apoptotic Interaction of Indole-3-Carbinol and Genistein with TRAIL on Endometrial Cancer Cells
- Paxilline enhances TRAIL-mediated apoptosis of glioma cells via modulation of c-FLIP, survivin and DR5
- Down-Regulation of Survivin by Nemadipine-A Sensitizes Cancer Cells to TRAIL-Induced Apoptosis