Diabetes Metab J.
2013 Jun;37(3):157-164. 10.4093/dmj.2013.37.3.157.
Neonatal Diabetes Caused by Activating Mutations in the Sulphonylurea Receptor
- Affiliations
-
- 1Henry Wellcome Centre for Gene Function, Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, UK. peter.proks@dpag.ox.ac.uk
- KMID: 2174288
- DOI: http://doi.org/10.4093/dmj.2013.37.3.157
Abstract
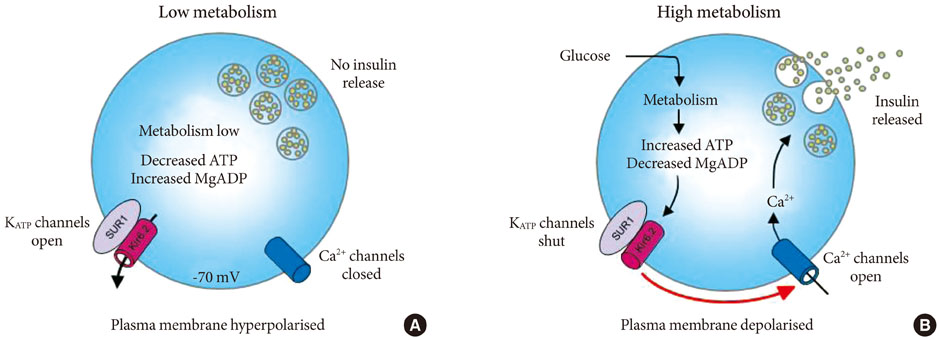
- Adenosine triphosphate (ATP)-sensitive potassium (KATP) channels in pancreatic beta-cells play a crucial role in insulin secretion and glucose homeostasis. These channels are composed of two subunits: a pore-forming subunit (Kir6.2) and a regulatory subunit (sulphonylurea receptor-1). Recent studies identified large number of gain of function mutations in the regulatory subunit of the channel which cause neonatal diabetes. Majority of mutations cause neonatal diabetes alone, however some lead to a severe form of neonatal diabetes with associated neurological complications. This review focuses on the functional effects of these mutations as well as the implications for treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Temple IK, James RS, Crolla JA, Sitch FL, Jacobs PA, Howell WM, Betts P, Baum JD, Shield JP. An imprinted gene(s) for diabetes? Nat Genet. 1995; 9:110–112.2. Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007; 56:1930–1937.3. Gloyn AL, Reimann F, Girard C, Edghill EL, Proks P, Pearson ER, Temple IK, Mackay DJ, Shield JP, Freedenberg D, Noyes K, Ellard S, Ashcroft FM, Gribble FM, Hattersley AT. Relapsing diabetes can result from moderately activating mutations in KCNJ11. Hum Mol Genet. 2005; 14:925–934.4. Greeley SA, Tucker SE, Worrell HI, Skowron KB, Bell GI, Philipson LH. Update in neonatal diabetes. Curr Opin Endocrinol Diabetes Obes. 2010; 17:13–19.5. Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003; 81:133–176.6. Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989; 54:87–143.7. Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005; 115:2047–2058.8. Inagaki N, Gonoi T, Clement JP 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995; 270:1166–1170.9. Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995; 377:338–344.10. Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997; 387:179–183.11. Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP 4th, Boyd AE 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995; 268:423–426.12. Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in KATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997; 16:1145–1152.13. Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP 4th, Gonzalez G, Aguilar-Bryan L, Permutt MA, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996; 272:1785–1787.14. Aittoniemi J, Fotinou C, Craig TJ, de Wet H, Proks P, Ashcroft FM. Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci. 2009; 364:257–267.15. Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, Larkin B, Colclough K, Hattersley AT, Ashcroft FM, Ellard S. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006; 15:1793–1800.16. Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, Scharfmann R, Bryan J, Aguilar-Bryan L, Vaxillaire M, Froguel P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006; 355:456–466.17. Patch AM, Flanagan SE, Boustred C, Hattersley AT, Ellard S. Mutations in the ABCC8 gene encoding the SUR1 subunit of the KATP channel cause transient neonatal diabetes, permanent neonatal diabetes or permanent diabetes diagnosed outside the neonatal period. Diabetes Obes Metab. 2007; 9:Suppl 2. 28–39.18. Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet. 2007; 81:375–382.19. Flanagan SE, Clauin S, Bellanne-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, Ellard S. Update of mutations in the genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009; 30:170–180.20. Zwaveling-Soonawala N, Hagebeuk EE, Slingerland AS, Ris-Stalpers C, Vulsma T, van Trotsenburg AS. Successful transfer to sulfonylurea therapy in an infant with developmental delay, epilepsy and neonatal diabetes (DEND) syndrome and a novel ABCC8 gene mutation. Diabetologia. 2011; 54:469–471.21. Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Stoy J, Steiner DF, Philipson LH, Bell GI, Hattersley AT, Ellard S. Neonatal Diabetes International Collaborative Group. Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008; 57:1034–1042.22. Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11. Rev Endocr Metab Disord. 2010; 11:193–198.23. Fatehi M, Raja M, Carter C, Soliman D, Holt A, Light PE. The ATP-sensitive K+ channel ABCC8 S1369A type 2 diabetes risk variant increases MgATPase activity. Diabetes. 2012; 61:241–249.24. Lin YW, Akrouh A, Hsu Y, Hughes N, Nichols CG, De Leon DD. Compound heterozygous mutations in the SUR1 (ABCC 8) subunit of pancreatic KATP channels cause neonatal diabetes by perturbing the coupling between Kir6.2 and SUR1 subunits. Channels (Austin). 2012; 6:133–138.25. Proks P, Ashcroft FM. Modeling KATP channel gating and its regulation. Prog Biophys Mol Biol. 2009; 99:7–19.26. Proks P, Shimomura K, Craig TJ, Girard CA, Ashcroft FM. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum Mol Genet. 2007; 16:2011–2019.27. Babenko AP, Vaxillaire M. Mechanism of KATP hyperactivity and sulfonylurea tolerance due to a diabetogenic mutation in L0 helix of sulfonylurea receptor 1 (ABCC8). FEBS Lett. 2011; 585:3555–3559.28. Zhou Q, Garin I, Castano L, Argente J, Munoz-Calvo MT, Perez de Nanclares G, Shyng SL. Neonatal diabetes caused by mutations in sulfonylurea receptor 1: interplay between expression and Mg-nucleotide gating defects of ATP-sensitive potassium channels. J Clin Endocrinol Metab. 2010; 95:E473–E478.29. de Wet H, Rees MG, Shimomura K, Aittoniemi J, Patch AM, Flanagan SE, Ellard S, Hattersley AT, Sansom MS, Ashcroft FM. Increased ATPase activity produced by mutations at arginine-1380 in nucleotide-binding domain 2 of ABCC8 causes neonatal diabetes. Proc Natl Acad Sci U S A. 2007; 104:18988–18992.30. Mannikko R, Flanagan SE, Sim X, Segal D, Hussain K, Ellard S, Hattersley AT, Ashcroft FM. Mutations of the same conserved glutamate residue in NBD2 of the sulfonylurea receptor 1 subunit of the KATP channel can result in either hyperinsulinism or neonatal diabetes. Diabetes. 2011; 60:1813–1822.31. Ortiz D, Voyvodic P, Gossack L, Quast U, Bryan J. Two neonatal diabetes mutations on transmembrane helix 15 of SUR1 increase affinity for ATP and ADP at nucleotide binding domain 2. J Biol Chem. 2012; 287:17985–17995.32. de Wet H, Proks P, Lafond M, Aittoniemi J, Sansom MS, Flanagan SE, Pearson ER, Hattersley AT, Ashcroft FM. A mutation (R826W) in nucleotide-binding domain 1 of ABCC8 reduces ATPase activity and causes transient neonatal diabetes. EMBO Rep. 2008; 9:648–654.33. Masia R, De Leon DD, MacMullen C, McKnight H, Stanley CA, Nichols CG. A mutation in the TMD0-L0 region of sulfonylurea receptor-1 (L225P) causes permanent neonatal diabetes mellitus (PNDM). Diabetes. 2007; 56:1357–1362.34. Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol. 1997; 504(Pt 1):35–45.35. Abdelmoneim AS, Hasenbank SE, Seubert JM, Brocks DR, Light PE, Simpson SH. Variations in tissue selectivity amongst insulin secretagogues: a systematic review. Diabetes Obes Metab. 2012; 14:130–138.36. Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002; 51:Suppl 3. S368–S376.37. Shield JP, Flanagan SE, Mackay DJ, Harries LW, Proks P, Girard C, Ashcroft FM, Temple IK, Ellard S. Mosaic paternal uniparental isodisomy and an ABCC8 gene mutation in a patient with permanent neonatal diabetes and hemihypertrophy. Diabetes. 2008; 57:255–258.38. Hambrock A, Loffler-Walz C, Quast U. Glibenclamide binding to sulphonylurea receptor subtypes: dependence on adenine nucleotides. Br J Pharmacol. 2002; 136:995–1004.39. Rafiq M, Flanagan SE, Patch AM, Shields BM, Ellard S, Hattersley AT. Neonatal Diabetes International Collaborative Group. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008; 31:204–209.40. Klupa T, Kowalska I, Wyka K, Skupien J, Patch AM, Flanagan SE, Noczynska A, Arciszewska M, Ellard S, Hattersley AT, Sieradzki J, Mlynarski W, Malecki MT. Mutations in the ABCC8 (SUR1 subunit of the KATP channel) gene are associated with a variable clinical phenotype. Clin Endocrinol (Oxf). 2009; 71:358–362.41. Vaxillaire M, Dechaume A, Busiah K, Cave H, Pereira S, Scharfmann R, de Nanclares GP, Castano L, Froguel P, Polak M. SUR1-Neonatal Diabetes Study Group. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes. 2007; 56:1737–1741.42. Stanik J, Gasperikova D, Paskova M, Barak L, Javorkova J, Jancova E, Ciljakova M, Hlava P, Michalek J, Flanagan SE, Pearson E, Hattersley AT, Ellard S, Klimes I. Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers. J Clin Endocrinol Metab. 2007; 92:1276–1282.43. Codner E, Flanagan SE, Ugarte F, Garcia H, Vidal T, Ellard S, Hattersley AT. Sulfonylurea treatment in young children with neonatal diabetes: dealing with hyperglycemia, hypoglycemia, and sick days. Diabetes Care. 2007; 30:e28–e29.44. Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, Slingerland AS, Shield J, Robert JJ, Holst JJ, Clark PM, Ellard S, Sovik O, Polak M, Hattersley AT. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006; 355:467–477.45. Remedi MS, Kurata HT, Scott A, Wunderlich FT, Rother E, Kleinridders A, Tong A, Bruning JC, Koster JC, Nichols CG. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of KATP-induced neonatal diabetes mellitus. Cell Metab. 2009; 9:140–151.46. Kumaraguru J, Flanagan SE, Greeley SA, Nuboer R, Stoy J, Philipson LH, Hattersley AT, Rubio-Cabezas O. Tooth discoloration in patients with neonatal diabetes after transfer onto glibenclamide: a previously unreported side effect. Diabetes Care. 2009; 32:1428–1430.47. Shah B, Breidbart E, Pawelczak M, Lam L, Kessler M, Franklin B. Improved long-term glucose control in neonatal diabetes mellitus after early sulfonylurea allergy. J Pediatr Endocrinol Metab. 2012; 25:353–356.48. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853.49. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–986.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Monogenic diabetes mellitus and clinical implications of genetic diagnosis
- A homozygous mutation in the insulin gene (INS) causing autosomal recessive neonatal diabetes in Saudi families
- Transient neonatal diabetes mellitus caused by a de novo ABCC8 gene mutation
- Successful sulfonylurea treatment in a patient with permanent neonatal diabetes mellitus with a novel KCNJ11 mutation
- Clinical and molecular characteristics of infantile-onset diabetes mellitus in Egypt