Diabetes Metab J.
2014 Aug;38(4):285-293. 10.4093/dmj.2014.38.4.285.
Assessment of Diabetic Polyneuropathy and Autonomic Neuropathy Using Current Perception Threshold in Korean Patients with Diabetes Mellitus
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. mkmoon@snu.ac.kr
- 2Department of Internal Medicine, Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2174105
- DOI: http://doi.org/10.4093/dmj.2014.38.4.285
Abstract
- BACKGROUND
The current perception threshold (CPT) could be quantified by stimulating Abeta and C fibers at 2,000 and 5 Hz, respectively. C fibers play a role in the autonomic nervous system and are involved in temperature and pain sensation. We evaluated the usefulness of CPT for diagnosing distal polyneuropathy (DPN) and cardiovascular autonomic neuropathy (CAN) in diabetic patients.
METHODS
The CPT was measured in the index finger (C7 level) and in the third toe (L5 level) in diabetic patients aged 30 to 69 years. We assessed DPN according to the neuropathy total symptom score-6 (NTSS-6) and 10-g monofilament pressure sensation. Subjects with a NTSS-6 >6 or with abnormal 10-g monofilament sensation were defined to have DPN. CAN was evaluated by spectral analysis of heart rate variability and by Ewing's traditional tests.
RESULTS
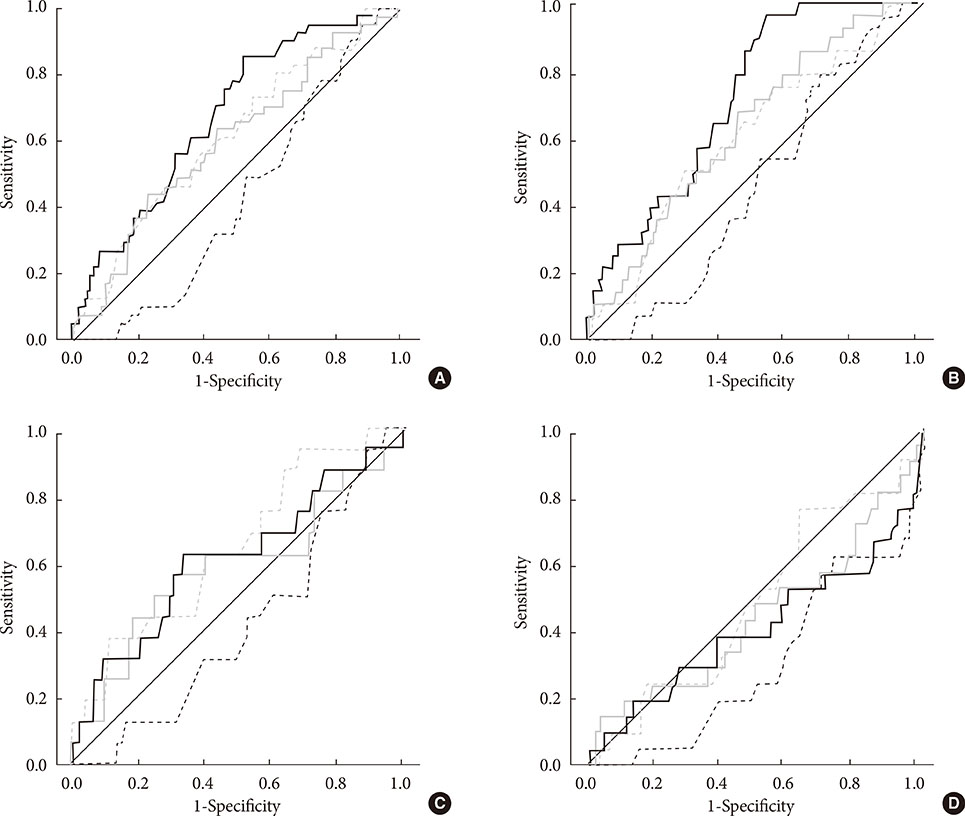
The subjects with DPN had significantly higher CPT at all of the frequencies than the subjects without DPN (P<0.05). Abnormal 10-g monofilament sensation and NTSS-6 >6 could be most precisely predicted by CPT at 2,000 and 5 Hz, respectively. However, only 6.5% and 19.6% of subjects with DPN had an abnormal CPT at 2,000 Hz at the C7 and L5 levels. Although CPT at 5 Hz showed a negative correlation with the power of low and high frequency in the spectral analysis (P<0.05), only 16.7% of subjects with CAN exhibited an abnormal CPT at the same frequency.
CONCLUSION
Although the CPT is significantly associated with neuropathic symptoms or signs corresponding to the nerve fiber stimulated, it provides little additional information compared with conventional evaluations.
MeSH Terms
Figure
Cited by 1 articles
-
Patterns of Nerve Conduction Abnormalities in Patients with Type 2 Diabetes Mellitus According to the Clinical Phenotype Determined by the Current Perception Threshold
Joong Hyun Park, Jong Chul Won
Diabetes Metab J. 2018;42(6):519-528. doi: 10.4093/dmj.2018.0068.
Reference
-
1. Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D. American Academy of Neurology. American Association of Neuromuscular and Electrodiagnostic Medicine. American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011; 76:1758–1765.2. American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care. 2013; 36:Suppl 1. S11–S66.3. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005; 28:956–962.4. Rendell MS, Dovgan DJ, Bergman TF, O'Donnell GP, Drobny EP, Katims JJ. Mapping diabetic sensory neuropathy by current perception threshold testing. Diabetes Care. 1989; 12:636–640.5. Pitei DL, Watkins PJ, Stevens MJ, Edmonds ME. The value of the neurometer in assessing diabetic neuropathy by measurement of the current perception threshold. Diabet Med. 1994; 11:872–876.6. Cheng WY, Jiang YD, Chuang LM, Huang CN, Heng LT, Wu HP, Tai TY, Lin BJ. Quantitative sensory testing and risk factors of diabetic sensory neuropathy. J Neurol. 1999; 246:394–398.7. Nather A, Neo SH, Chionh SB, Liew SC, Sim EY, Chew JL. Assessment of sensory neuropathy in diabetic patients without diabetic foot problems. J Diabetes Complications. 2008; 22:126–131.8. Matsutomo R, Takebayashi K, Aso Y. Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. J Int Med Res. 2005; 33:442–453.9. Masson EA, Veves A, Fernando D, Boulton AJ. Current perception thresholds: a new, quick, and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia. 1989; 32:724–728.10. Bastyr EJ 3rd, Price KL, Bril V. MBBQ Study Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther. 2005; 27:1278–1294.11. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996; 93:1043–1065.12. Neurotron, Incorporated Innovative Medical Technology: Neurometer® CPT® Painless Electrodiagnostic Clinical and Laboratory Sensory Nerve Testing Equipment. updated 2013 Dec 2. Available from: http://www.neurotron.com/downloads/Professional_Feasibility_Report_Neurology.pdf.13. Veves A, Young MJ, Manes C, Boulton AJ. Differences in peripheral and autonomic nerve function measurements in painful and painless neuropathy. A clinical study. Diabetes Care. 1994; 17:1200–1202.14. Centers for Disease Control and Prevention: Age-adjusted incidence of end-stage renal disease related to diabetes mellitus (ESRD-DM) per 100,000 diabetic population, by race, ethnicity, and sex, United States, 1980-2008. updated 2013 Oct 21. Available from: http://www.cdc.gov/diabetes/statistics/esrd/fig5.htm.15. Liu Z, Fu C, Wang W, Xu B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients: a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes. 2010; 8:62.16. Legrady P, Bajcsi D, Lengyel C, Varkonyi TT, Fejes I, Kempler P, Abraham G. Investigation of cardiac autonomic and peripheral sensory neuropathy in diabetic and nondiabetic patients with hypertension. Clin Exp Hypertens. 2013; 35:465–469.17. Keresztes K, Istenes I, Folhoffer A, Lakatos PL, Horvath A, Csak T, Varga P, Kempler P, Szalay F. Autonomic and sensory nerve dysfunction in primary biliary cirrhosis. World J Gastroenterol. 2004; 10:3039–3043.18. Putz Z, Tabak AG, Toth N, Istenes I, Nemeth N, Gandhi RA, Hermanyi Z, Keresztes K, Jermendy G, Tesfaye S, Kempler P. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care. 2009; 32:181–183.19. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–2293.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment Strategies for Diabetic Neuropathy
- Clinical spectrum and diagnosis of diabetic neuropathies
- Comparisons between Several Neurologic Tests of Large Myelinated Nerves in Type 2 Diabetic Patients with Peripheral Polyneuropathy
- Relationship between Cardiovascular Autonomic Neuropathy and Diabetic Retinopathy in Patients with Non-Insulin Dependent Diabetes Mellitus
- Usefulness of Questionnaires, Physical Examination and Median Mixed Nerve Conduction Studies in Patients with Diabetes Mellitus