Diabetes Metab J.
2014 Dec;38(6):472-479. 10.4093/dmj.2014.38.6.472.
GDF15 Is a Novel Biomarker for Impaired Fasting Glucose
- Affiliations
-
- 1Research Center for Endocrine and Metabolic Diseases, Chungnam National University Hospital, Daejeon, Korea. minhos@cnu.ac.kr, bonjeong@cnu.ac.kr
- 2Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 3Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
- 4Department of Family Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 2174085
- DOI: http://doi.org/10.4093/dmj.2014.38.6.472
Abstract
- BACKGROUND
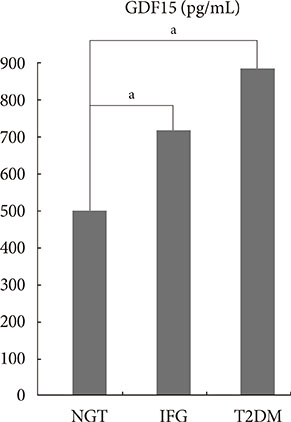
Growth differentiation factor-15 (GDF15) is a protein that belongs to the transforming growth factor beta superfamily. An elevated serum level of GDF15 was found to be associated with type 2 diabetes mellitus (T2DM). T2DM is an inflammatory disease that progresses from normal glucose tolerance (NGT) to impaired fasting glucose (IFG). Hence, we aimed to validate the relationship between GDF15 and IFG.
METHODS
The participants were divided into the following three groups: NGT (n=137), IFG (n=29), and T2DM (n=75). The controls and T2DM outpatients visited the hospital for routine health check-ups. We used fasting blood glucose to detect IFG in nondiabetic patients. We checked the body mass index (BMI), C-reactive protein level, metabolic parameters, and fasting serum GDF15 level.
RESULTS
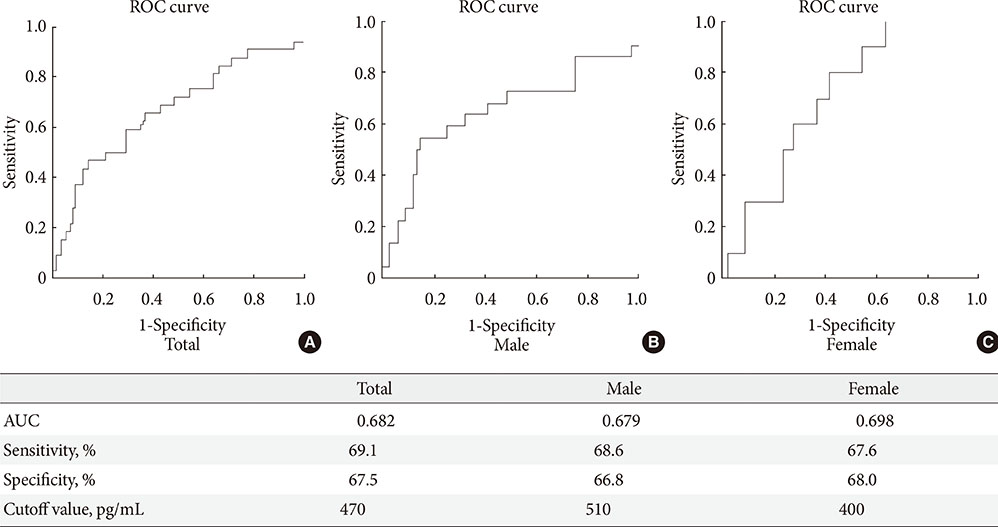
Age, BMI, triglyceride, insulin, glucose, homeostatic model assessment-insulin resistance (HOMA-IR), and GDF15 levels were elevated in the IFG and T2DM groups compared to the NGT group. In the correlation analysis between metabolic parameters and GDF15, age and HOMA-IR had a significant positive correlation with GDF15 levels. GDF15 significantly discriminated between IFG and NGT, independent of age, BMI, and HOMA-IR. The serum levels of GDF15 were more elevated in men than in women. As a biomarker for IFG based on the receiver operating characteristic curve analysis, the cutoff value of GDF15 was 510 pg/mL in males and 400 pg/mL in females.
CONCLUSION
GDF15 had a positive correlation with IR independent of age and BMI, and the serum level of GDF15 was increased in the IFG and T2DM groups. GDF15 may be a novel biomarker for detecting IFG in nondiabetic patients.
Keyword
MeSH Terms
-
Biomarkers
Blood Glucose
Body Mass Index
C-Reactive Protein
Diabetes Mellitus, Type 2
Fasting*
Female
Glucose*
Growth Differentiation Factor 15
Humans
Insulin
Male
Outpatients
Prediabetic State
ROC Curve
Transforming Growth Factor beta
Triglycerides
Blood Glucose
C-Reactive Protein
Glucose
Growth Differentiation Factor 15
Insulin
Transforming Growth Factor beta
Figure
Cited by 1 articles
-
Deterioration of Sleep Quality According to Glycemic Status
Myung Haeng Hur, Mi-Kyoung Lee, Kayeon Seong, Jun Hwa Hong
Diabetes Metab J. 2020;44(5):679-686. doi: 10.4093/dmj.2019.0125.
Reference
-
1. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention;2011.2. Korea Centers for Disease Control and Prevention (KCDC) and the Korean Ministry of Health and Welfare: Diabetes fact sheet in Korea 2012. updated 2014 Oct 8. Available from: http://www.diabetes.or.kr/temp/diabetes_factsheet_2013111.pdf.3. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.4. Hanefeld M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc Diabetol. 2007; 6:20.5. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–1350.6. Goldberg R, Temprosa M, Otvos J, Brunzell J, Marcovina S, Mather K, Arakaki R, Watson K, Horton E, Barrett-Connor E. Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2013; 98:3989–3998.7. Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999; 353:1649–1652.8. Coban E, Sari R, Ozdogan M, Akcit F. Levels of plasma fibrinogen and d-dimer in patients with impaired fasting glucose. Exp Clin Endocrinol Diabetes. 2005; 113:35–37.9. Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998; 92:593–596.10. Festa A, D'Agostino R Jr, Tracy RP, Haffner SM. Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002; 51:1131–1137.11. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011; 11:98–107.12. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999; 22:233–240.13. Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004; 164:2147–2155.14. Deedwania PC, Fonseca VA. Diabetes, prediabetes, and cardiovascular risk: shifting the paradigm. Am J Med. 2005; 118:939–947.15. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, Breit SN. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997; 94:11514–11519.16. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995; 95:2409–2415.17. Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009; 150:1688–1696.18. Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, Wollert KC. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol. 2012; 167:671–678.19. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999; 22:920–924.20. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37:Suppl 1. S81–S90.21. Bergman M. Inadequacies of absolute threshold levels for diagnosing prediabetes. Diabetes Metab Res Rev. 2010; 26:3–6.22. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–553.23. Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability: does it matter? Endocr Rev. 2010; 31:171–182.24. Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol. 2008; 295:H2495–H2502.25. Rohlfing C, Wiedmeyer HM, Little R, Grotz VL, Tennill A, England J, Madsen R, Goldstein D. Biological variation of glycohemoglobin. Clin Chem. 2002; 48:1116–1118.26. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009; 1:9–17.27. Sato KK, Hayashi T, Harita N, Yoneda T, Nakamura Y, Endo G, Kambe H. Combined measurement of fasting plasma glucose and A1C is effective for the prediction of type 2 diabetes: the Kansai Healthcare Study. Diabetes Care. 2009; 32:644–646.28. Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM. A1C between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010; 33:2104–2109.29. Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007; 53:284–291.30. Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys Acta. 1997; 1354:40–44.31. Lawton LN, Bonaldo MF, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, de Jesus GM, Wellington S, Knowles JA, Warburton D, Brown S, Soares MB. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997; 203:17–26.32. Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, Thompson DD. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998; 273:13760–13767.33. Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005; 67:356–364.34. Tajiri Y, Mimura K, Umeda F. High-sensitivity C-reactive protein in Japanese patients with type 2 diabetes. Obes Res. 2005; 13:1810–1816.35. Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, Kavalkova P, Matoulek M, Kasalicky M, Haluzik M. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009; 161:397–404.36. Karczewska-Kupczewska M, Kowalska I, Nikolajuk A, Adamska A, Otziomek E, Gorska M, Straczkowski M. Hyperinsulinemia acutely increases serum macrophage inhibitory cytokine-1 concentration in anorexia nervosa and obesity. Clin Endocrinol (Oxf). 2012; 76:46–50.37. Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006; 236:13–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: GDF15 Is a Novel Biomarker for Impaired Fasting Glucose (Diabetes Metab J 2014;38:472-9)
- Response: GDF15 Is a Novel Biomarker for Impaired Fasting Glucose (Diabetes Metab J 2014;38:472-9)
- Prevalence and Risk Factors for Diabetes Mellitus and Impaired Fasting Glucose of Adults
- The Role of Growth Differentiation Factor 15 in Energy Metabolism
- Risk Factors of Impaired Fasting Glucose and Type 2 Diabetes Mellitus: Using Datamining