J Gynecol Oncol.
2008 Dec;19(4):251-255. 10.3802/jgo.2008.19.4.251.
Comparison between L and E gene amplification analytical methods for human papillomavirus typing
- Affiliations
-
- 1Department of Biological Engineering, SeoKyeong University, Korea.
- 2Department of Obstetrics and Gynecology, Hanyang University College of Medicine, Seoul, Korea. kimkt@hanyanguniv.ac.kr
- KMID: 2173415
- DOI: http://doi.org/10.3802/jgo.2008.19.4.251
Abstract
OBJECTIVE
L and E6/E7 gene amplification analyses were compared to identify human papillomavirus (HPV) infection and verify the HPV type, with the intent to minimize HPV typing errors.
METHODS
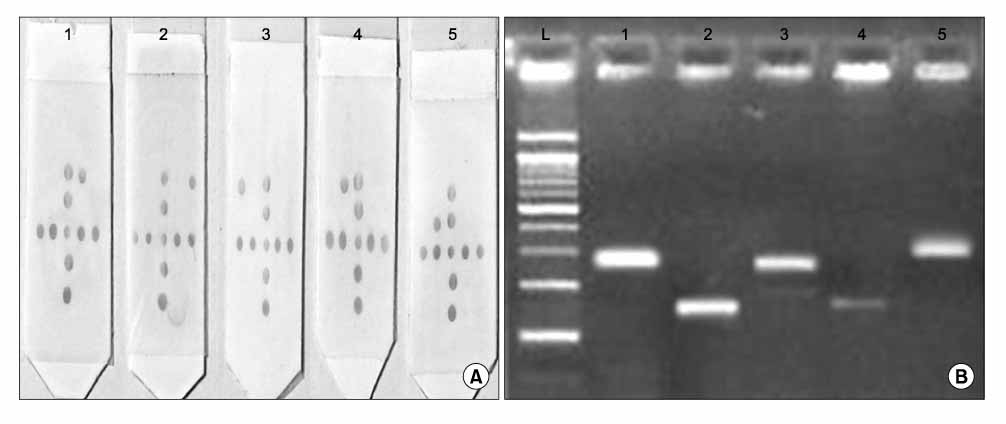
L1 gene verified HPV typing was accomplished via polymerase chain reaction (PCR) and membrane assays. Verification of HPV typing via E6/E7 genes was accomplished through nested multiplexed PCR. The results from 104 samples were compared.
RESULTS
The rates of accordance and difference were 35% and 65%, respectively. For 29% of the analyses, nested multiplexed PCR was more diversified than the membrane assay.
CONCLUSION
HPV can be classified into low-risk HPV and high-risk HPV groups. In parallel amplifications of the L and E genes is more efficient for accurate diagnosis in light of the different symptoms and attendant precautions of the risk groups.
Keyword
Figure
Reference
-
1. Ferlay J, Bray P, Pizani P, Parkin DM. Globocan 2002: Cancer incidence, mortality and prevalence worldwide. 2004. Lyon: IARC Press.2. Khanna N, Phillips MD. Adherence to care plan in women with abnormal Papanicolaou smears: A review of barriers and interventions. J Am Board Fam Pract. 2001. 14:123–130.3. Castellsagué X, Muñoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis - role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr. 2003. 31:20–28.4. Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002. 55:244–265.5. Muñoz N, Bosch FX, Castellsagué X, Díaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004. 111:278–285.6. von Krogh G. Management of anogenital warts (condylomata acuminata). Eur J Dermatol. 2001. 11:598–603.7. Prendiville W, Davies P. Prendiville W, Davis P, editors. The human papillomavirus. The health professionals HPV handbook: Human papillomavirus and cervical cancer. 2004. Oxford: Taylor and Francis;11–26.8. Luxton J, Shepherd P. Human papillomavirus antigens and T-cell recognition. Curr Opin Infect Dis. 2001. 14:139–143.9. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004. 324:17–27.10. Smits HL, Tieben LM, Tjong-A-Hung SP, Jebbink MF, Minnaar RP, Jansen CL, et al. Detection and typing of human papillomaviruses present in fixed and stained archival cervical smears by a consensus polymerase chain reaction and direct sequence analysis allow the identification of a broad spectrum of human papillomavirus types. J Gen Virol. 1992. 73:3263–3268.11. Lungu O, Sun XW, Felix J, Richart RM, Silverstein S, Wright TC Jr. Relationship of human papillomavirus type to grade of cervical intraepithelial neoplasia. JAMA. 1992. 267:2493–2496.12. Van Den Brule AJ, Walboomers JM, Du Maine M, Kenemans P, Meijer CJ. Difference in prevalence of human papillomavirus genotypes in cytomorphologically normal cervical smears is associated with a history of cervical intraepithelial neoplasia. Int J Cancer. 1991. 48:404–408.13. Jacobs MV, de Roda Husman AM, van den Brule AJ, Snijders PJ, Meijer CJ, Walboomers JM. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. J Clin Microbiol. 1995. 33:901–905.14. Sasagawa T, Minemoto Y, Basha W, Yamazaki H, Nakamura M, Yoshimoto H, et al. A new PCR-based assay amplifies the E6-E7 genes of most mucosal human papillomaviruses (HPV). Virus Res. 2000. 67:127–139.15. Sotlar K, Diemer D, Dethleffs A, Hack Y, Stubner A, Vollmer N, et al. Detection and typing of human papillomavirus by e6 nested multiplex PCR. J Clin Microbiol. 2004. 42:3176–3184.16. Longworth MS, Laimins LA. Pathogenesis of human papilloviruses in differentiating epithelia. Microbiol Mol Biol Rev. 2004. 68:362–372.17. Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002. 89:213–228.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Validation Study for the Sex Typing Using Amelogenin
- The Disaster Victim Identification using DNA Typing
- Study on p53 Mutation and MDM2 Amplification in Cervical Cancer
- Detection and typing of human papillomavirus DNA by PCR using consensus primers in various cervical lesions of Korean women
- Comparison of the Novel Human Papillomavirus 4 Auto-capillary Electrophoresis Test with the Hybrid Capture 2 Assay and with the PCR HPV Typing Set Test in the Detection of High-Risk HPV Including HPV 16 and 18 Genotypes in Cervical Specimens