Blood Res.
2013 Dec;48(4):287-291. 10.5045/br.2013.48.4.287.
Nodular lymphoid hyperplasia of the stomach in a patient with multiple submucosal tumors
- Affiliations
-
- 1Department of Internal Medicine, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Gastroenterology, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Pathology, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea. hanpathol@naver.com
- 4Department of Laboratory Medicine, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2172877
- DOI: http://doi.org/10.5045/br.2013.48.4.287
Abstract
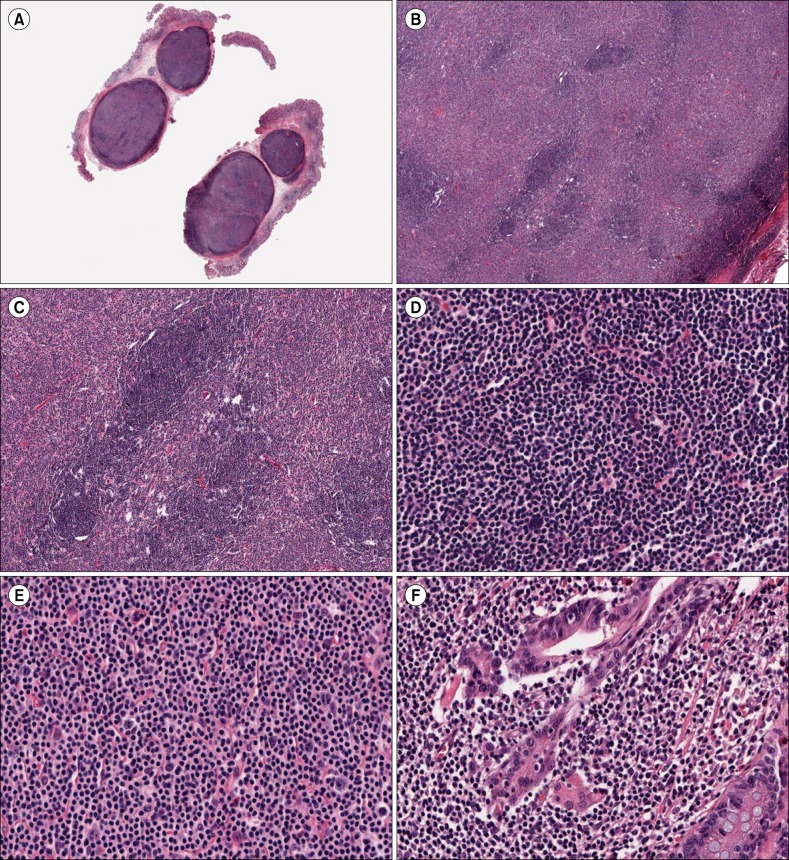
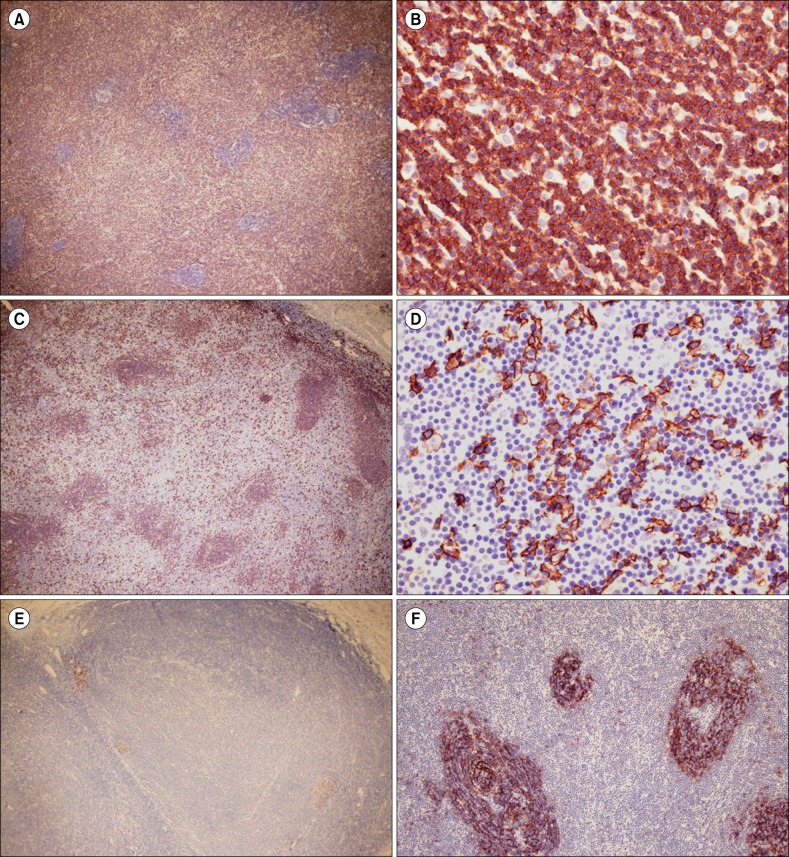
- Nodular lymphoid hyperplasia of the stomach is a rare lymphoproliferative disorder. Here, we report a 38-year-old man who presented with multiple submucosal tumors of the stomach. Histologically, the lesions were characterized by multiple discrete submucosal nodules of lymphoid cells. The infiltrates between the lymphoid follicles were composed mainly of medium-sized lymphoid cells with abundant clear cytoplasm, as well as a few large cells with vesicular nuclei. The gastric mucosa exhibited multifocal lymphoid aggregates and some of the epithelial cells were infiltrated by small lymphocytes mimicking lymphoepithelial lesions. Histopathology was consistent with mucosa-associated lymphoid tissue lymphoma. However, the infiltrating lymphoid cells were positive for CD2, CD3, CD5, and CD7. In addition, polymerase chain reaction analysis of the immunoglobulin heavy chain and T-cell receptor gene rearrangements demonstrated polyclonality. This case was diagnosed as reactive lymphoid hyperplasia of the stomach.
Keyword
MeSH Terms
Figure
Reference
-
1. Abbondanzo SL, Sobin LH. Gastric "pseudolymphoma": a retrospective morphologic and immunophenotypic study of 97 cases. Cancer. 1997; 79:1656–1663. PMID: 9128979.2. Banks PM. Gastrointestinal lymphoproliferative disorders. Histopathology. 2007; 50:42–54. PMID: 17204020.
Article3. Ranchod M, Lewin KJ, Dorfman RF. Lymphoid hyperplasia of the gastrointestinal tract. A study of 26 cases and review of the literature. Am J Surg Pathol. 1978; 2:383–400. PMID: 736212.4. Kojima M, Nakamura N, Itoh H, et al. Histological variety of localized lymphoid hyperplasia of the large intestine: histopathological, immunohistochemical and genotypic findings of 16 cases. J Clin Exp Hematop. 2009; 49:15–21. PMID: 19474513.
Article5. Remstein ED, Dogan A, Einerson RR, et al. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006; 30:1546–1553. PMID: 17122510.
Article6. Wundisch T, Thiede C, Morgner A, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005; 23:8018–8024. PMID: 16204012.7. Nakamura S, Ye H, Bacon CM, et al. Clinical impact of genetic aberrations in gastric MALT lymphoma: a comprehensive analysis using interphase fluorescence in situ hybridisation. Gut. 2007; 56:1358–1363. PMID: 17525089.
Article8. Wang G, Auerbach A, Wei M, et al. t(11;18)(q21;q21) in extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue in stomach: a study of 48 cases. Mod Pathol. 2009; 22:79–86. PMID: 18820661.
Article9. Hummel M, Oeschger S, Barth TF, et al. Wotherspoon criteria combined with B cell clonality analysis by advanced polymerase chain reaction technology discriminates covert gastric marginal zone lymphoma from chronic gastritis. Gut. 2006; 55:782–787. PMID: 16423889.
Article10. Genta RM, Hamner HW, Graham DY. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993; 24:577–583. PMID: 8505036.
Article11. Chen XY, Liu WZ, Shi Y, Zhang DZ, Xiao SD, Tytgat GN. Helicobacter pylori associated gastric diseases and lymphoid tissue hyperplasia in gastric antral mucosa. J Clin Pathol. 2002; 55:133–137. PMID: 11865009.
Article12. Tomita S, Kojima M, Imura J, et al. Diffuse nodular lymphoid hyperplasia of the large bowel without hypogammaglobulinemia or malabsorption syndrome: a case report and literature review. Int J Surg Pathol. 2002; 10:297–302. PMID: 12490983.13. Khuroo MS, Khuroo NS, Khuroo MS. Diffuse duodenal nodular lymphoid hyperplasia: a large cohort of patients etiologically related to Helicobacter pylori infection. BMC Gastroenterol. 2011; 11:36. PMID: 21481240.
Article14. Rubio-Tapia A, Hernandez-Calleros J, Trinidad-Hernandez S, Uscanga L. Clinical characteristics of a group of adults with nodular lymphoid hyperplasia: a single center experience. World J Gastroenterol. 2006; 12:1945–1948. PMID: 16610004.
Article15. Ryan JC. Premalignant conditions of the small intestine. Semin Gastrointest Dis. 1996; 7:88–93. PMID: 8705262.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Small Intestinal Nodular Lymphoid Hyperplasia
- A Case of Primary Jejunal Malignant Lymphoma Associated with Nodular Lymphoid Hyperplasia
- Lymphoid hyperplasia of the stomach manifesting as umbilicated polypoid lesions : report of two cases
- Surgical Treatment of the Pulmonary Nodular Lymphoid Hyperplasia: A case report
- Pulmonary Nodular Lymphoid Hyperplasia in a 33-Year-Old Woman