Blood Res.
2015 Jun;50(2):73-79. 10.5045/br.2015.50.2.73.
Apoptosis: role in myeloid cell development
- Affiliations
-
- 1Stem Cells and Haematological Disorders Laboratory, Department of Biochemistry, School of Life Sciences, University of Hyderabad, Hyderabad, India. guttiravi@gmail.com
- 2Department of Biotechnology, GITAM Institute of Science, GITAM University, Visakhapatnam, India.
- KMID: 2172770
- DOI: http://doi.org/10.5045/br.2015.50.2.73
Abstract
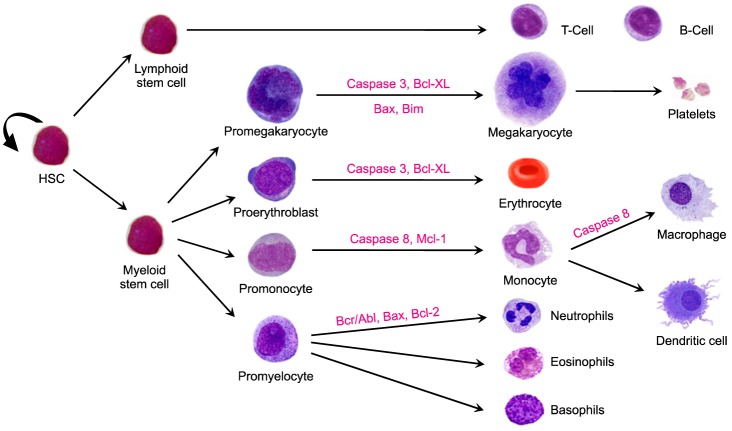
- Hematopoiesis is the process that generates blood cells in an organism from the pluripotent stem cells. Hematopoietic stem cells are characterized by their ability to undergo self-renewal and differentiation. The self-renewing ability ensures that these pluripotent cells are not depleted from the bone marrow niche. A proper balance between cell death and cell survival is necessary to maintain a homeostatic condition, hence, apoptosis, or programmed cell death, is an essential step in hematopoiesis. Recent studies, however, have introduced a new aspect to this process, citing the significance of the apoptosis mediator, caspase, in cell development and differentiation. Extensive research has been carried out to study the possible role of caspases and other apoptosis related factors in the developmental processes. This review focuses on the various apoptotic factors involved in the development and differentiation of myeloid lineage cells: erythrocytes, megakaryocytes, and macrophages.
Keyword
MeSH Terms
Figure
Reference
-
1. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972; 26:239–257. PMID: 4561027.
Article2. Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997; 22:299–306. PMID: 9270303.
Article3. Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993; 75:653–660. PMID: 8242741.4. Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003; 22:8543–8567. PMID: 14634618.
Article5. Yi CH, Yuan J. The Jekyll and Hyde functions of caspases. Dev Cell. 2009; 16:21–34. PMID: 19154716.
Article6. Miura M. Active participation of cell death in development and organismal homeostasis. Dev Growth Differ. 2011; 53:125–136. PMID: 21338339.
Article7. Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999; 96:10964–10967. PMID: 10500109.
Article8. Nadiri A, Wolinski MK, Saleh M. The inflammatory caspases: key players in the host response to pathogenic invasion and sepsis. J Immunol. 2006; 177:4239–4245. PMID: 16982854.
Article9. Zermati Y, Garrido C, Amsellem S, et al. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001; 193:247–254. PMID: 11208865.
Article10. Allombert-Blaise C, Tamiji S, Mortier L, et al. Terminal differentiation of human epidermal keratinocytes involves mitochondria- and caspase-dependent cell death pathway. Cell Death Differ. 2003; 10:850–852. PMID: 12815468.
Article11. Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A. 2002; 99:11025–11030. PMID: 12177420.
Article12. Wride MA. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos Trans R Soc Lond B Biol Sci. 2011; 366:1219–1233. PMID: 21402582.
Article13. Sordet O, Rébé C, Plenchette S, et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002; 100:4446–4453. PMID: 12393560.
Article14. Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000; 407:784–788. PMID: 11048729.
Article15. Bellone M, Iezzi G, Rovere P, et al. Processing of engulfed apoptotic bodies yields T cell epitopes. J Immunol. 1997; 159:5391–5399. PMID: 9548479.17. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001; 104:487–501. PMID: 11239407.18. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998; 281:1305–1308. PMID: 9721089.
Article19. Bhardwaj A, Aggarwal BB. Receptor-mediated choreography of life and death. J Clin Immunol. 2003; 23:317–332. PMID: 14601641.20. Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998; 95:570–575. PMID: 9435233.
Article21. Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991; 66:233–243. PMID: 1713127.
Article22. Walczak H, Degli-Esposti MA, Johnson RS, et al. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J. 1997; 16:5386–5397. PMID: 9311998.
Article23. Tan KB, Harrop J, Reddy M, et al. Characterization of a novel TNF-like ligand and recently described TNF ligand and TNF receptor superfamily genes and their constitutive and inducible expression in hematopoietic and non-hematopoietic cells. Gene. 1997; 204:35–46. PMID: 9434163.
Article24. Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995; 14:5579–5588. PMID: 8521815.
Article25. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004; 116:205–219. PMID: 14744432.26. Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001; 292:727–730. PMID: 11326099.
Article27. Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008; 27(Suppl 1):S2–S19. PMID: 19641503.
Article28. Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990; 348:334–336. PMID: 2250705.
Article29. Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993; 53:4701–4714. PMID: 8402648.30. Nagata Y, Nagahisa H, Aida Y, Okutomi K, Nagasawa T, Todokoro K. Thrombopoietin induces megakaryocyte differentiation in hematopoietic progenitor FDC-P2 cells. J Biol Chem. 1995; 270:19673–19675. PMID: 7649975.
Article31. Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008; 132:631–644. PMID: 18295580.
Article32. Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000; 191:253–264. PMID: 10637270.33. Bárcena A, Park SW, Banapour B, Muench MO, Mechetner E. Expression of Fas/CD95 and Bcl-2 by primitive hematopoietic progenitors freshly isolated from human fetal liver. Blood. 1996; 88:2013–2025. PMID: 8822920.34. Nuñez G, Clarke MF. The Bcl-2 family of proteins: regulators of cell death and survival. Trends Cell Biol. 1994; 4:399–403. PMID: 14731816.
Article35. Motoyama N, Kimura T, Takahashi T, Watanabe T, Nakano T. bcl-x prevents apoptotic cell death of both primitive and definitive erythrocytes at the end of maturation. J Exp Med. 1999; 189:1691–1698. PMID: 10359572.
Article36. Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003; 426:671–676. PMID: 14668867.
Article37. Gobbi G, Mirandola P, Carubbi C, et al. Phorbol ester-induced PKCepsilon down-modulation sensitizes AML cells to TRAIL-induced apoptosis and cell differentiation. Blood. 2009; 113:3080–3087. PMID: 18988868.38. Gregory CJ, Eaves AC. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978; 51:527–537. PMID: 623913.
Article39. Rylski M, Welch JJ, Chen YY, et al. GATA-1-mediated proliferation arrest during erythroid maturation. Mol Cell Biol. 2003; 23:5031–5042. PMID: 12832487.
Article40. Dolznig H, Habermann B, Stangl K, et al. Apoptosis protection by the Epo target Bcl-X(L) allows factor-independent differentiation of primary erythroblasts. Curr Biol. 2002; 12:1076–1085. PMID: 12121614.
Article41. Silva M, Grillot D, Benito A, Richard C, Nuñez G, Fernéndez-Luna JL. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-XL and Bcl-2. Blood. 1996; 88:1576–1582. PMID: 8781412.
Article42. Gregoli PA, Bondurant MC. The roles of Bcl-X(L) and apopain in the control of erythropoiesis by erythropoietin. Blood. 1997; 90:630–640. PMID: 9226163.
Article43. Gregory T, Yu C, Ma A, Orkin SH, Blobel GA, Weiss MJ. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood. 1999; 94:87–96. PMID: 10381501.
Article44. Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999; 98:181–191. PMID: 10428030.45. Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007; 445:102–105. PMID: 17167422.
Article46. Zeuner A, Eramo A, Testa U, et al. Control of erythroid cell production via caspase-mediated cleavage of transcription factor SCL/Tal-1. Cell Death Differ. 2003; 10:905–913. PMID: 12867998.
Article47. Osada M, Komeno T, Todokoro K, et al. Immature megakaryocytes undergo apoptosis in the absence of thrombopoietin. Exp Hematol. 1999; 27:131–138. PMID: 9923451.
Article48. Patel SR, Hartwig JH, Italiano JE Jr. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005; 115:3348–3354. PMID: 16322779.
Article49. De Botton S, Sabri S, Daugas E, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002; 100:1310–1317. PMID: 12149212.
Article50. Clarke MC, Savill J, Jones DB, Noble BS, Brown SB. Compart-mentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol. 2003; 160:577–587. PMID: 12591916.
Article51. Kozuma Y, Kojima H, Yuki S, Suzuki H, Nagasawa T. Continuous expression of Bcl-xL protein during megakaryopoiesis is post-translationally regulated by thrombopoietin-mediated Akt activation, which prevents the cleavage of Bcl-xL. J Thromb Haemost. 2007; 5:1274–1282. PMID: 17389006.
Article52. Acarin L, Villapol S, Faiz M, Rohn TT, Castellano B, González B. Caspase-3 activation in astrocytes following postnatal excitotoxic damage correlates with cytoskeletal remodeling but not with cell death or proliferation. Glia. 2007; 55:954–965. PMID: 17487878.
Article53. Kozuma Y, Yuki S, Ninomiya H, Nagasawa T, Kojima H. Caspase activation is involved in early megakaryocyte differentiation but not in platelet production from megakaryocytes. Leukemia. 2009; 23:1080–1086. PMID: 19212331.
Article54. Melloni E, Secchiero P, Celeghini C, et al. Functional expression of TRAIL and TRAIL-R2 during human megakaryocytic development. J Cell Physiol. 2005; 204:975–982. PMID: 15828026.
Article55. Kozuma Y, Ninomiya H, Murata S, Kono T, Mukai HY, Kojima H. The pro-apoptotic BH3-only protein Bim regulates cell cycle progression of hematopoietic progenitors during megakaryopoiesis. J Thromb Haemost. 2010; 8:1088–1097. PMID: 20128868.
Article56. White MJ, Schoenwaelder SM, Josefsson EC, et al. Caspase-9 mediates the apoptotic death of megakaryocytes and platelets, but is dispensable for their generation and function. Blood. 2012; 119:4283–4290. PMID: 22294729.
Article57. Josefsson EC, Burnett DL, Lebois M, et al. Platelet production proceeds independently of the intrinsic and extrinsic apoptosis pathways. Nat Commun. 2014; 5:3455. PMID: 24632563.
Article58. Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-kappaB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol. 2000; 20:8855–8865. PMID: 11073986.59. Perlman H, Pagliari LJ, Georganas C, Mano T, Walsh K, Pope RM. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J Exp Med. 1999; 190:1679–1688. PMID: 10587358.
Article60. Liu H, Perlman H, Pagliari LJ, Pope RM. Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-kappaB, Bad, or caspase activation. J Exp Med. 2001; 194:113–126. PMID: 11457886.61. Rébé C, Cathelin S, Launay S, et al. Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood. 2007; 109:1442–1450. PMID: 17047155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alterations of the Apoptosis Genes and Their Products in Non-small Cell Lung Cancer Tissues
- The Pharmacological Inhibition of ERK5 Enhances Apoptosis in Acute Myeloid Leukemia Cells
- Role of Autophagy in the Control of Cell Death and Inflammation
- Effect of G-CSF on Myeloid Leukemic Cell Lines after Treatment with Ara-C
- Bcl-2 Expression in Korean Fetal Lungs