Chonnam Med J.
2010 Apr;46(1):7-18. 10.4068/cmj.2010.46.1.7.
Type 3 Repeats of Thrombospondin-2 Increases Metastasis in Mouse Colorectal Cancer CT-26 Cells
- Affiliations
-
- 1Department of Pathology, Chonnam National University Medical School, Gwangju, Korea. cchoi@chonnam.ac.kr
- 2Department of Surgery, Chonnam National University Medical School, Gwangju, Korea.
- 3Research Institute of Chonnam National University, Gwangju, Korea.
- 4Department of Biological Science, Sungkyunkwan University, Suwon, Korea.
- KMID: 2172251
- DOI: http://doi.org/10.4068/cmj.2010.46.1.7
Abstract
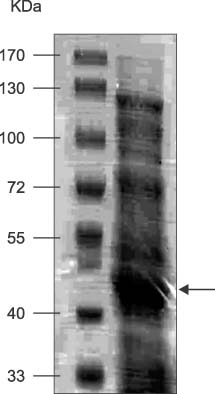
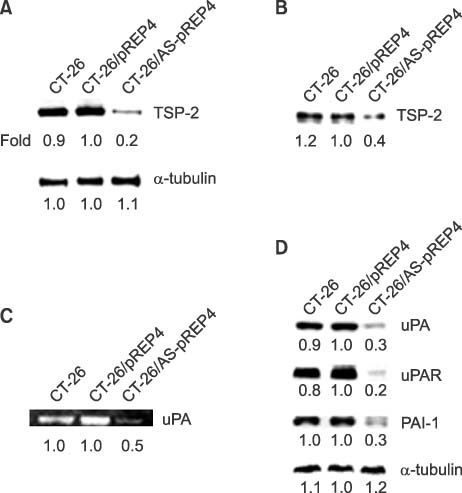
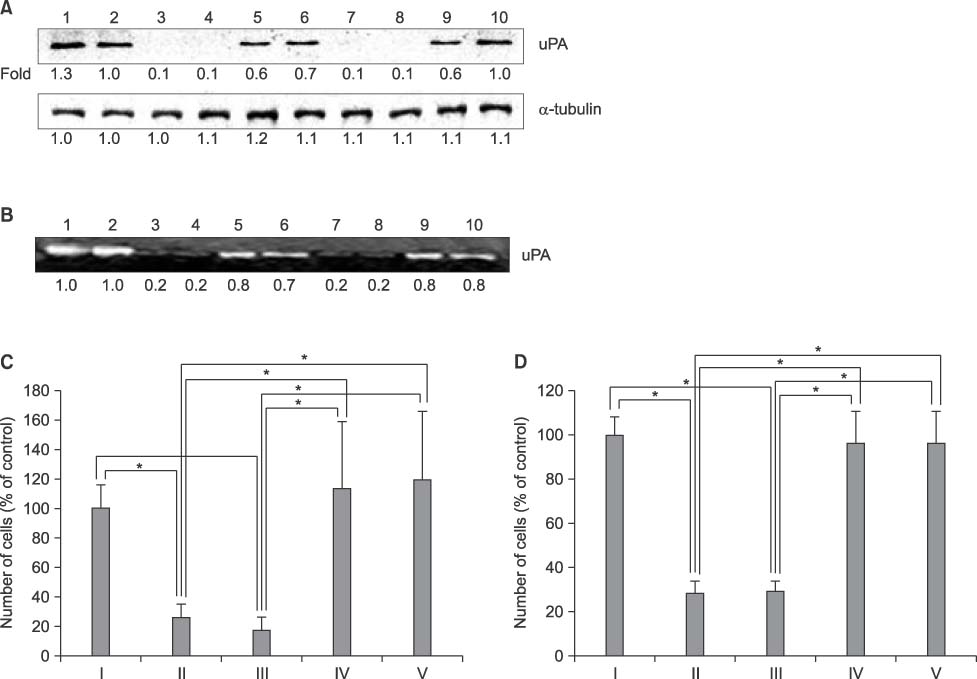
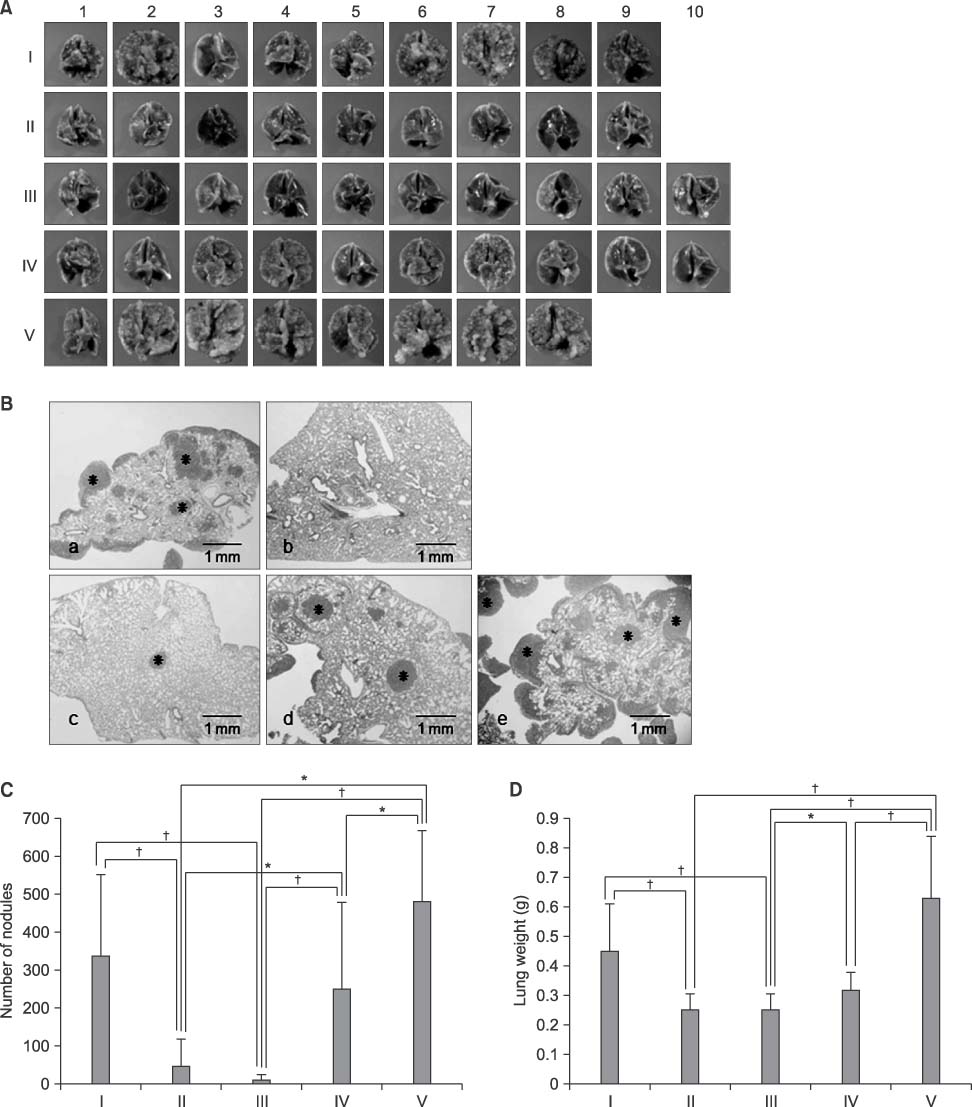
- Thrombospondins (TSPs) are secreted multimeric glycoproteins that modulate extracellular matrix structure and cell behaviour. TSPs are involved in platelet aggregation, cell adhesion, migration, angiogenesis, wound healing, and proliferation. The purpose of this study was to investigate the function of TSP-2 on the invasion and migration of CT-26, and to determine the domain that is responsible for these functions. The antisense cDNA of TSP-2 was transfected to CT-26 (CT-26/AS-pREP4), which underexpressed TSP-2. Reduction of TSP-2 expression resulted in decreased activity, and decreased expression of the plasminogen/plasmin system. All of which resulted in decreased invasion and migration in vitro. The panels of murine TSP-2 cDNA subunits in CT-26/AS-pREP4 were established. The transfectants containing type 3 repeats domain revealed an increased in vitro invasion and migration, and increased pulmonary metastasis when inoculated into the tail vein of a syngenic mouse. The type 3 repeats domain was both integrin alphavbeta3 and phosphatidylinositol (PI) 3-kinase/Akt dependent. These findings may be useful for the development of therapeutic interventions that block either integrin alphavbeta3 or PI 3-kinase dependent Akt phosphorylation, resulting in the reduction of plasminogen/plasmin system and consequently block cell invasion, migration, and metastatic spread of colon cancer cells.
MeSH Terms
-
Animals
Cell Adhesion
Colonic Neoplasms
Colorectal Neoplasms
DNA, Complementary
Extracellular Matrix
Glycoproteins
Integrin alphaVbeta3
Mice
Neoplasm Invasiveness
Neoplasm Metastasis
Phosphatidylinositol 3-Kinases
Phosphatidylinositols
Phosphorylation
Platelet Aggregation
Thrombospondins
Veins
Wound Healing
DNA, Complementary
Glycoproteins
Integrin alphaVbeta3
Phosphatidylinositol 3-Kinases
Phosphatidylinositols
Thrombospondins
Figure
Reference
-
1. Bae JM, Jung KW, Won YJ. Estimation of cancer deaths in Korea for the upcoming years. J Korean Med Sci. 2002. 17:611–615.
Article2. Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004. 36:961–968.
Article3. O'Rourke KM, Laherty CD, Dixit VM. Thrombospondin 1 and thrombospondin 2 are expressed as both homo- and heterotrimers. J Biol Chem. 1992. 267:24921–24924.4. Sargiannidou I, Qiu C, Tuszynski GP. Mechanisms of thrombospondin-1-mediated metastasis and angiogenesis. Semin Thromb Hemost. 2004. 30:127–136.
Article5. Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002. 6:1–12.
Article6. Yee KO, Connolly CM, Duquette M, Kazerounian S, Washington R, Lawler J. The effect of thrombospondin-1 on breast cancer metastasis. Breast Cancer Res Treat. 2009. 114:85–96.
Article7. Albo D, Berger DH, Wang TN, Hu X, Rothman V, Tuszynski GP. Thrombospondin-1 and transforming growth factor-beta l promote breast tumor cell invasion through up-regulation of the plasminogen/plasmin system. Surgery. 1997. 122:493–500.
Article8. Arnoletti JP, Albo D, Granick MS, Solomon MP, Castiglioni A, Rothman VL, et al. Thrombospondin and transforming growth factor-beta 1 increase expression of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 in human MDA-MB-231 breast cancer cells. Cancer. 1995. 76:998–1005.
Article9. Albo D, Berger DH, Vogel J, Tuszynski GP. Thrombospondin-1 and transforming growth factor beta-1 upregulate plasminogen activator inhibitor type 1 in pancreatic cancer. J Gastrointest Surg. 1999. 3:411–417.
Article10. Albo D, Arnoletti JP, Castiglioni A, Granick MS, Solomon MP, Rothman VL, et al. Thrombospondin (TSP) and transforming growth factor beta 1 (TGF-beta) promote human A549 lung carcinoma cell plasminogen activator inhibitor type 1 (PAI-1) production and stimulate tumor cell attachment in vitro. Biochem Biophys Res Commun. 1994. 203:857–865.
Article11. Yabkowitz R, Mansfield PJ, Dixit VM, Suchard SJ. Motility of human carcinoma cells in response to thrombospondin: relationship to metastatic potential and thrombospondin structural domains. Cancer Res. 1993. 53:378–387.12. Bai W, Wang L, Ji W, Gao H. Expression profiling of supraglottic carcinoma: PTEN and thrombospondin 2 are associated with inhibition of lymphatic metastasis. Acta Otolaryngol. 2008. 129:569–574.
Article13. Urquidi V, Sloan D, Kawai K, Agarwal D, Woodman AC, Tarin D, et al. Contrasting expression of thrombospondin-1 and osteopontin correlates with absence or presence of metastatic phenotype in an isogenic model of spontaneous human breast cancer metastasis. Clin Cancer Res. 2002. 8:61–74.14. Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, Frazier WA, Roberts DD, Steeg PS. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994. 54:6504–6511.15. Huet E, Brassart B, Cauchard JH, Debelle L, Birembaut P, Wallach J, et al. Cumulative influence of elastin peptides and plasminogen on matrix metalloproteinase activation and type I collagen invasion by HT-1080 fibrosarcoma cells. Clin Exp Metastasis. 2002. 19:107–117.16. Kobayashi H, Gonda T, Uetake H, Higuchi T, Enomoto M, Sugihara K. JTE-522, a selective COX-2 inhibitor, interferes with the growth of lung metastases from colorectal cancer in rats due to inhibition of neovascularization: a vascular cast model study. Int J Cancer. 2004. 112:920–926.
Article17. Anilkumar N, Annis DS, Mosher DF, Adams JC. Trimeric assembly of the C-terminal region of thrombospondin-1 or thrombospondin-2 is necessary for cell spreading and fascin spike organisation. J Cell Sci. 2002. 115:2357–2366.
Article18. Lymn JS, Patel MK, Clunn GF, Rao SJ, Gallagher KL, Hughes AD. Thrombospondin-1 differentially induces chemotaxis and DNA synthesis of human venous smooth muscle cells at the receptor-binding level. J Cell Sci. 2002. 115:4353–4360.
Article19. Adams J, Lawler J. Extracellular matrix: the thrombospondin family. Curr Biol. 1993. 3:188–190.20. Bonnefoy A, Hantgan R, Legrand C, Frojmovic MM. A model of platelet aggregation involving multiple interactions of thrombospondin-1, fibrinogen, and GPIIbIIIa receptor. J Biol Chem. 2001. 276:5605–5612.
Article21. Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992. 90:1513–1522.
Article22. Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996. 12:697–715.
Article23. Lawler J, Weinstein R, Hynes RO. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988. 107:2351–2361.
Article24. Lymn JS, Rao SJ, Clunn GF, Gallagher KL, O'Neil C, Thompson NT, et al. Phosphatidylinositol 3-kinase and focal adhesion kinase are early signals in the growth factor-like responses to thrombospondin-1 seen in human vascular smooth muscle. Arterioscler Thromb Vasc Biol. 1999. 19:2133–2140.
Article25. Sliva D, Rizzo MT, English D. Phosphatidylinositol 3-kinase and NF-kappaB regulate motility of invasive MDA-MB-231 human breast cancer cells by the secretion of urokinase-type plasminogen activator. J Biol Chem. 2002. 277:3150–3157.
Article26. Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991. 254:274–277.
Article27. Morgan H, Hill PA. Human breast cancer cell-mediated bone collagen degradation requires plasminogen activation and matrix metalloproteinase activity. Cancer Cell Int. 2005. 5:1.28. Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, et al. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci U S A. 1999. 96:14888–14893.
Article29. Dawson DW, Volpert OV, Pearce SF, Schneider AJ, Silverstein RL, Henkin J, et al. Three distinct D-amino acid substitutions confer potent antiangiogenic activity on an inactive peptide derived from a thrombospondin-1 type 1 repeat. Mol Pharmacol. 1999. 55:332–338.
Article30. Asch AS, Liu I, Briccetti FM, Barnwell JW, Kwakye-Berko F, Dokun A, et al. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science. 1993. 262:1436–1440.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic diagnosis and treatment of early colorectal cancer
- Endotracheal Metastasis Seen on FDG PET/CT in a Patient with Previous Colorectal Cancer
- Expression of Tumor Metastasis Related Genes in Korean Colorectal Cancers and Cell lines
- Thrombospondin-1 and Inhibition of Tumor Growth
- Sphingosylphosphorylcholine Induces Thrombospondin-1 Secretion in MCF10A Cells via ERK2