Chonnam Med J.
2014 Aug;50(2):45-51. 10.4068/cmj.2014.50.2.45.
Effects of Flavonoid Compounds on beta-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons
- Affiliations
-
- 1Department of Neurology, Chonnam National University Medical School, Gwangju, Korea. byeong.kim@jnu.ac.kr
- 2Department of Pharmacology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2172158
- DOI: http://doi.org/10.4068/cmj.2014.50.2.45
Abstract
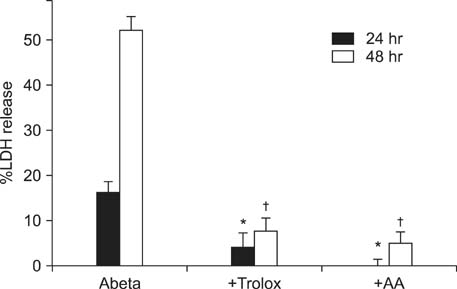
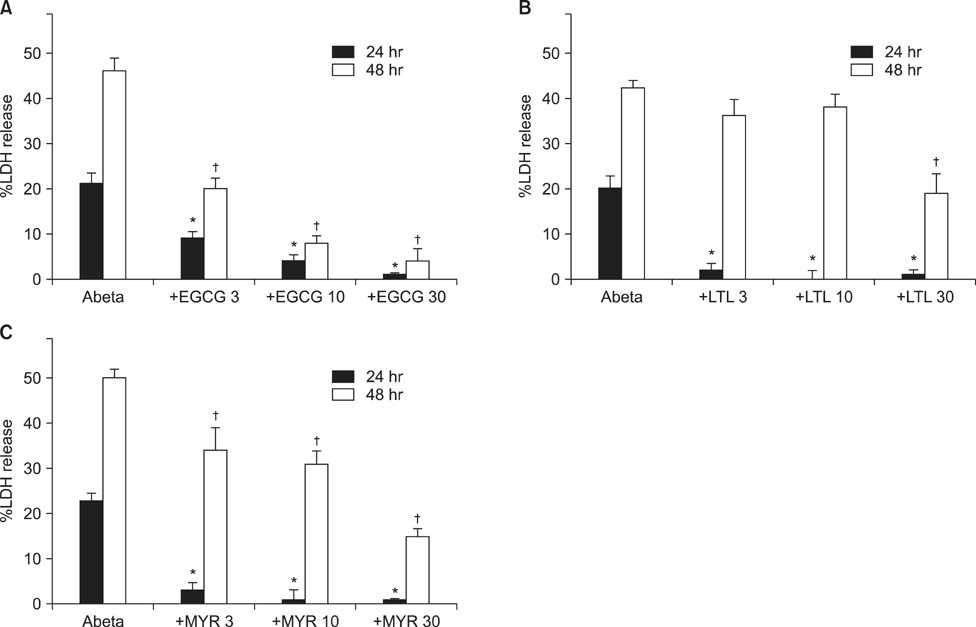
- Excessive accumulation of beta-amyloid peptide (Abeta) is one of the major mechanisms responsible for neuronal death in Alzheimer's disease. Flavonoids, primarily antioxidants, are a group of polyphenolic compounds synthesized in plant cells. The present study aimed to identify flavonoid compounds that could inhibit Abeta-induced neuronal death by examining the effects of various flavonoids on the neurotoxicity of Abeta fragment 25-35 (Abeta25-35) in mouse cortical cultures. Abeta25-35 induced concentration- and exposure-time-dependent neuronal death. Neuronal death induced by 20 microM Abeta25-35 was significantly inhibited by treatment with either Trolox or ascorbic acid. Among 10 flavonoid compounds tested [apigenin, baicalein, catechin, epicatechin, epigallocatechin gallate (EGCG), kaempferol, luteolin, myricetin, quercetin, and rutin], all except apigenin showed strong 1,1-diphenyl-2-pycrylhydrazyl (DPPH) scavenging activity under cell-free conditions. The flavonoid compounds except apigenin at a concentration of 30 microM also significantly inhibited neuronal death induced by 20 microM Abeta25-35 at the end of 24 hours of exposure. Epicatechin, EGCG, luteolin, and myricetin showed more potent and persistent neuroprotective action than did the other compounds. These results demonstrated that oxidative stress was involved in Abeta-induced neuronal death, and antioxidative flavonoid compounds, especially epicatechin, EGCG, luteolin, and myricetin, could inhibit neuronal death. These findings suggest that these four compounds may be developed as neuroprotective agents against Alzheimer's disease.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of NADPH Oxidase Inhibitors and Mitochondria-Targeted Antioxidants on Amyloid β1-42-Induced Neuronal Deaths in Mouse Mixed Cortical Cultures
Shinae Hwang, Jong-Keun Kim
Chonnam Med J. 2018;54(3):159-166. doi: 10.4068/cmj.2018.54.3.159.
Reference
-
1. Robards K, Antolovich M. Analytical chemistry of fruit bioflavonoids. A review. Analyst. 1997; 122:11R–34R.2. Lu MF, Xiao ZT, Zhang HY. Where do health benefits of flavonoids come from? Insights from flavonoid targets and their evolutionary history. Biochem Biophys Res Commun. 2013; 434:701–704.
Article3. Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 2003; 133:3248S–3254S.
Article4. Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006; 54:9966–9977.
Article5. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993; 342:1007–1011.
Article6. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996; 20:933–956.
Article7. Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000; 63:1035–1042.
Article8. Zeng LH, Zhang HD, Xu CJ, Bian YJ, Xu XJ, Xie QM, et al. Neuroprotective effects of flavonoids extracted from licorice on kainate-induced seizure in mice through their antioxidant properties. J Zhejiang Univ Sci B. 2013; 14:1004–1012.
Article9. Dugas AJ Jr, Castañeda-Acosta J, Bonin GC, Price KL, Fischer NH, Winston GW. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. J Nat Prod. 2000; 63:327–331.
Article10. Cho JG, Song NY, Nam TG, Shrestha S, Park HJ, Lyu HN, et al. Flavonoids from the grains of C1/R-S transgenic rice, the transgenic Oryza sativa spp. japonica, and their radical scavenging activities. J Agric Food Chem. 2013; 61:10354–10359.
Article11. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001; 74:418–425.
Article12. Thilakarathna SH, Rupasinghe HP. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013; 5:3367–3387.
Article13. Beking K, Vieira A. Flavonoid intake and disability-adjusted life years due to Alzheimer's and related dementias: a population-based study involving twenty-three developed countries. Public Health Nutr. 2010; 13:1403–1409.
Article14. Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007; 165:1364–1371.
Article15. Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, et al. Neurologic Diseases in the Elderly Research Group. Incidence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology. 2000; 54:11 Suppl 5. S10–S15.16. Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991; 30:572–580.
Article17. Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985; 82:4245–4249.
Article18. Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987; 325:733–736.
Article19. Small DH, McLean CA. Alzheimer's disease and the amyloid beta protein: What is the role of amyloid? J Neurochem. 1999; 73:443–449.20. Small DH, Mok SS, Bornstein JC. Alzheimer's disease and Abeta toxicity: from top to bottom. Nat Rev Neurosci. 2001; 2:595–598.
Article21. Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993; 13:1676–1687.
Article22. Jordán J, Galindo MF, Miller RJ. Role of calpain- and interleukin-1 beta converting enzyme-like proteases in the beta-amyloid-induced death of rat hippocampal neurons in culture. J Neurochem. 1997; 68:1612–1621.
Article23. Bastianetto S, Ramassamy C, Doré S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci. 2000; 12:1882–1890.
Article24. Bisaglia M, Venezia V, Piccioli P, Stanzione S, Porcile C, Russo C, et al. Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochem Int. 2002; 41:43–54.
Article25. Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995; 64:253–265.
Article26. Smid SD, Maag JL, Musgrave IF. Dietary polyphenol-derived protection against neurotoxic β-amyloid protein: from molecular to clinical. Food Funct. 2012; 3:1242–1250.
Article27. Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, et al. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994; 91:3270–3274.
Article28. Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. Beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992; 12:376–389.
Article29. Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987; 7:357–368.
Article30. Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991; 563:311–314.
Article31. Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987; 20:83–90.
Article32. Goodman Y, Steiner MR, Steiner SM, Mattson MP. Nordihydroguaiaretic acid protects hippocampal neurons against amyloid beta-peptide toxicity, and attenuates free radical and calcium accumulation. Brain Res. 1994; 654:171–176.
Article33. Bruce AJ, Malfroy B, Baudry M. beta-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci U S A. 1996; 93:2312–2316.
Article34. Okada Y, Okada M. Protective effects of plant seed extracts against amyloid β-induced neurotoxicity in cultured hippocampal neurons. J Pharm Bioallied Sci. 2013; 5:141–147.
Article35. Liu R, Zhang T, Yang H, Lan X, Ying J, Du G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-β25-35-induced toxicity in mice. J Alzheimers Dis. 2011; 24:85–100.
Article36. Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998; 55:1409–1415.
Article37. Drieu K. Preparation and definition of Ginkgo biloba extract. Presse Med. 1986; 15:1455–1457.
Article38. Moosmann B, Behl C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin Investig Drugs. 2002; 11:1407–1435.
Article39. Youdim KA, Spencer JP, Schroeter H, Rice-Evans C. Dietary flavonoids as potential neuroprotectants. Biol Chem. 2002; 383:503–519.
Article40. Gao J, Inagaki Y, Li X, Kokudo N, Tang W. Research progress on natural products from traditional Chinese medicine in treatment of Alzheimer's disease. Drug Discov Ther. 2013; 7:46–57.
Article41. Jones QR, Warford J, Rupasinghe HP, Robertson GS. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol Sci. 2012; 33:602–610.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of NADPH Oxidase Inhibitors and Mitochondria-Targeted Antioxidants on Amyloid βâ‚â‚‹â‚„â‚‚-Induced Neuronal Deaths in Mouse Mixed Cortical Cultures
- Protective effect of the aerial parts of Silybum marianum against amyloid β protein (25-35)-induced neuronal death in cultured neurons
- Anti-oxidative neuroprotection by estrogens in mouse cortical cultures
- Inhibitory effect of the leaves and stems of Actinidia arguta on Aβ (25–35)-induced neuronal cell death and memory impairment
- beta-Amyloid Neurotoxicity on Basal Forebrain Cholinergic Neuron Cultures