Chonnam Med J.
2015 Apr;51(1):26-32. 10.4068/cmj.2015.51.1.26.
Long-Term Outcome of Treatment with Topical Corticosteroids for Severe Dry Eye Associated with Sjogren's Syndrome
- Affiliations
-
- 1Department of Ophthalmology, Chonnam National University Medical School and Hospital, Gwangju, Korea. kcyoon@jnu.ac.kr
- 2Medical Research Center of Gene Regulation and Center for Creative Biomedical Scientists, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2172144
- DOI: http://doi.org/10.4068/cmj.2015.51.1.26
Abstract
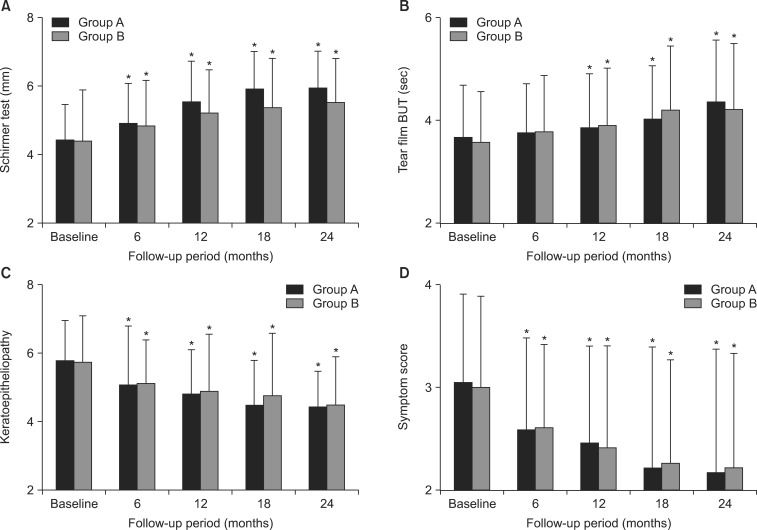
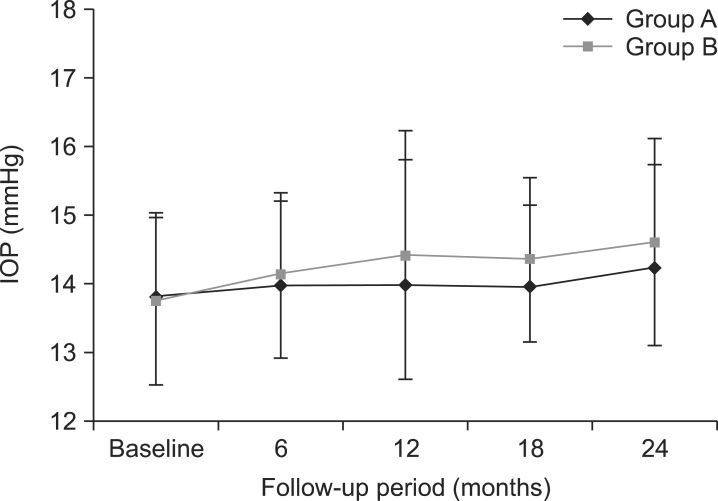
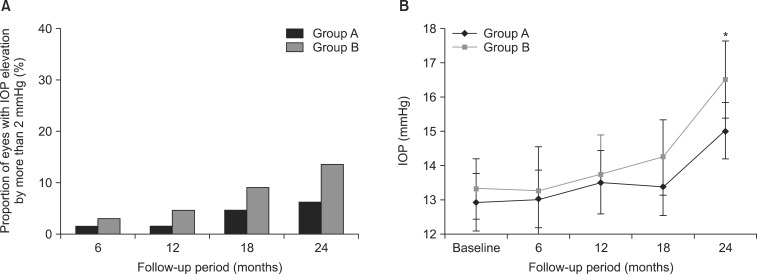
- This retrospective study was performed to analyze the long-term outcome of topical corticosteroid treatment for severe dry eye associated with Sjogren's syndrome (SS). Patients who had severe dry eye associated with SS were topically treated with loteprednol etabonate 0.5% (group A, n=66) or fluorometholone 0.1% (group B, n=67) twice daily and were followed up for 2 years. Visual acuity (VA), intraocular pressure (IOP), Schirmer test, tear film breakup time (BUT), keratoepitheliopathy, and symptom scores were measured at baseline and 6, 12, 18, and 24 months after treatment. VA and IOP were not changed significantly during follow-up in either group. Schirmer test results, keratoepitheliopathy, and symptom scores at 6, 12, 18, and 24 months (p<0.05) and tear film BUT at 12, 18, and 24 months (p<0.05) significantly improved after treatment compared with baseline in both groups. No significant differences between the groups were found in any parameter during follow-up. At 24 months, the number of patients with IOP elevation of more than 2 mmHg compared with baseline was 4 in group A (6.1%) and 9 in group B (13.4%). The mean IOP in these patients was lower in group A than in group B (15.00+/-0.82 mmHg versus 16.50+/-1.12 mmHg; p=0.04). Long-term application of low-dose topical corticosteroids is effective for controlling signs and symptoms of chronic, severe dry eye associated with SS. Loteprednol etabonate 0.5% may have a lower risk for IOP elevation than fluorometholone 0.1%.
MeSH Terms
Figure
Reference
-
1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003; 136:318–326. PMID: 12888056.2. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92. PMID: 17508116.3. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005; 353:1711–1723. PMID: 16236742.4. Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000; 11:478–483. PMID: 11141645.5. Nussenblatt RB, Palestine AG. Cyclosporine: immunology, pharmacology and therapeutic uses. Surv Ophthalmol. 1986; 31:159–169. PMID: 3544293.6. Hemady R, Tauber J, Foster CS. Immunosuppressive drugs in immune and inflammatory ocular disease. Surv Ophthalmol. 1991; 35:369–385. PMID: 2038720.7. Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorders. Br J Ophthalmol. 2005; 89:1363–1367. PMID: 16170133.8. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–639. PMID: 10768324.9. Liew MS, Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjogren's syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012; 96:1498–1503. PMID: 23001257.10. Goto E, Matsumoto Y, Kamoi M, Endo K, Ishida R, Dogru M, et al. Tear evaporation rates in Sjögren syndrome and non-Sjögren dry eye patients. Am J Ophthalmol. 2007; 144:81–85. PMID: 17509507.11. Horwath-Winter J, Berghold A, Schmut O, Floegel I, Solhdju V, Bodner E, et al. Evaluation of the clinical course of dry eye syndrome. Arch Ophthalmol. 2003; 121:1364–1368. PMID: 14557170.12. Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999; 106:811–816. PMID: 10201607.13. Gündüz K, Ozdemir O. Topical cyclosporin treatment of keratoconjunctivitis sicca in secondary Sjögren's syndrome. Acta Ophthalmol (Copenh). 1994; 72:438–442. PMID: 7825408.14. Aragona P, Spinella R, Rania L, Postorino E, Sommario MS, Roszkowska AM, et al. Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjögren syndrome. Eur J Ophthalmol. 2013; 23:368–376. PMID: 23225089.15. Aragona P, Stilo A, Ferreri F, Mobrici M. Effects of the topical treatment with NSAIDs on corneal sensitivity and ocular surface of Sjögren's syndrome patients. Eye (Lond). 2005; 19:535–539. PMID: 15184937.16. Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. Sjögren's International Collaborative Clinical Alliance (SICCA) Research Groups. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012; 64:475–487. PMID: 22563590.17. de Venecia G, Davis MD. Diurnal variation of intraocular pressure in the normal eye. Arch Ophthalmol. 1963; 69:752–757. PMID: 14026102.18. Yoon KC, Heo H, Im SK, You IC, Kim YH, Park YG. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007; 144:86–92. PMID: 17493572.19. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001; 108:586–592. PMID: 11237914.20. Kaido M, Goto E, Dogru M, Tsubota K. Punctal occlusion in the management of chronic Stevens-Johnson syndrome. Ophthalmology. 2004; 111:895–900. PMID: 15121365.21. Samudre SS, Lattanzio FA Jr, Williams PB, Sheppard JD Jr. Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther. 2004; 20:533–547. PMID: 15684812.22. Daynes RA, Araneo BA. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989; 19:2319–2325. PMID: 2606141.23. Linden M, Brattsand R. Effects of a corticosteroid, budesonide, on alveolar macrophage and blood monocyte secretion of cytokines: differential sensitivity of GM-CSF, IL-1 beta, and IL-6. Pulm Pharmacol. 1994; 7:43–47. PMID: 8003851.24. Avunduk AM, Avunduk MC, Varnell ED, Kaufman HE. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003; 136:593–602. PMID: 14516798.25. Bodor N, Buchwald P. Soft drug design: general principles and recent applications. Med Res Rev. 2000; 20:58–101. PMID: 10608921.26. Druzgala P, Hochhaus G, Bodor N. Soft drugs--10. Blanching activity and receptor binding affinity of a new type of glucocorticoid: loteprednol etabonate. J Steroid Biochem Mol Biol. 1991; 38:149–154. PMID: 2004037.27. Loteprednol Etabonate US Uveitis Study Group. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Am J Ophthalmol. 1999; 127:537–544. PMID: 10334346.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence and Clinical Aspects of Sjogren Syndrome in Dry Eye Patients
- Therapeutic Effect of Topical Testosterone Gel in Patients with Dry Eye Syndrome
- Assessment of the Compliance with 0.1% Cyclosporine A in Dry-Eye Patients with Sjögren's Syndrome
- Effects of Carbomer Eye Gels on the Ocular Surface in Dry Eye Patients
- Efficacy and Safety of Topical Unpreserved 0.1% Fluorometholone Ophthalmic Solution on Dry Eye Syndrome