Ann Dermatol.
2011 Aug;23(3):313-320. 10.5021/ad.2011.23.3.313.
Expression of Pro-inflammatory Protein S100A12 (EN-RAGE) in Behcet's Disease and Its Association with Disease Activity: A Pilot Study
- Affiliations
-
- 1Department of Dermatology and Cutaneous Biology Research Institute, Seoul, Korea. dbang@yuhs.ac
- 2Specialization Research Center, Hallym University Hangang Sacred Heart Hospital, Seoul, Korea.
- 3Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2171922
- DOI: http://doi.org/10.5021/ad.2011.23.3.313
Abstract
- BACKGROUND
S100A12 is a member of the S100 family of calcium-binding proteins and is secreted either in inflamed tissues or in the bloodstream by activated neutrophils. Expression of S100A12 has been reported in various diseases, especially non-infectious inflammatory diseases, such as Kawasaki disease, giant cell arteritis and inflammatory bowel disease.
OBJECTIVE
This study was conducted to determine both the tissue expression and the serum levels of S100A12 in Behcet's disease (BD) patients and the correlation of the S100A12 serum level with disease activity of BD.
METHODS
We included in this study ten BD patients who fulfilled the criteria for diagnosis, according to the International Study Group for BD. The activity of BD was calculated using the BD Current Activity Form. The serum concentrations of both S100A12 and interleukin-8 were measured by the enzyme-linked immunosorbent assay, before and after treatment. Immunohistochemical studies were also performed to detect S100A12 expression in the skin.
RESULTS
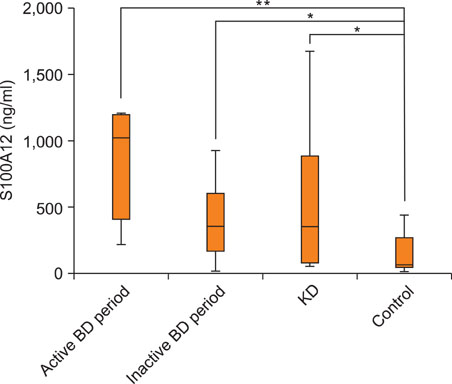
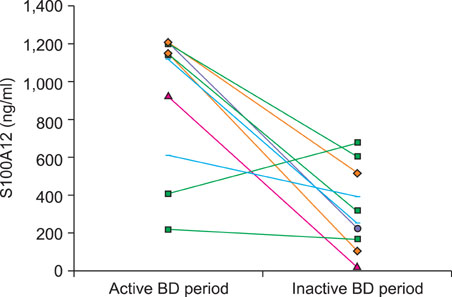
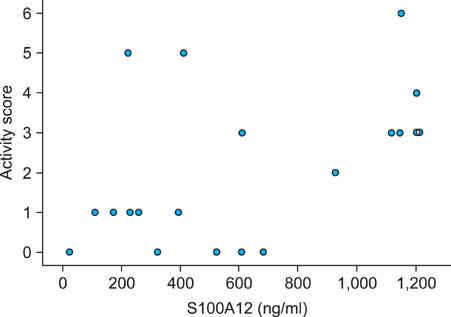
The serum S100A12 level was significantly increased in the active BD period (p<0.001), in the inactive BD period (p=0.041) and in patients with active Kawasaki disease (p=0.028), compared with the serum level in the healthy controls. The serum S100A12 level decreased significantly from baseline, compared to post-treatment (p=0.017). The activity score of BD was significantly correlated with the serum level of S100A12 (Spearman's coefficient=0.464, p=0.039). Immunohistochemical studies showed that S100A12 was strongly expressed in the erythema nodosum-like skin lesions of patients.
CONCLUSION
S100A12 contributes to the pathogenesis of BD related to neutrophil hyperactivity and reflects the disease activity in BD patients.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
New Insights in the Clinical Understanding of Behçet's Disease
Sung Bin Cho, Suhyun Cho, Dongsik Bang
Yonsei Med J. 2012;53(1):35-42. doi: 10.3349/ymj.2012.53.1.35.
Reference
-
1. Zouboulis CC. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Adamantiades-Behçet disease. Fitzpatrick's dermatology in general medicine. 2007. 7th ed. New York: McGraw-Hill;1620–1626.2. James DG. Behcet's syndrome. N Engl J Med. 1979. 301:431–432.3. Al-Otaibi LM, Porter SR, Poate TW. Behçet's disease: a review. J Dent Res. 2005. 84:209–222.
Article4. Kalayciyan A, Zouboulis C. An update on Behçet's disease. J Eur Acad Dermatol Venereol. 2007. 21:1–10.
Article5. Chun SI, Su WP, Lee S, Rogers RS 3rd. Erythema nodosum-like lesions in Behçet's syndrome: a histopathologic study of 30 cases. J Cutan Pathol. 1989. 16:259–265.
Article6. Demirkesen C, Tüzüner N, Mat C, Senocak M, Büyükbabani N, Tüzün Y, et al. Clinicopathologic evaluation of nodular cutaneous lesions of Behçet syndrome. Am J Clin Pathol. 2001. 116:341–346.
Article7. Jorizzo JL, Abernethy JL, White WL, Mangelsdorf HC, Zouboulis CC, Sarica R, et al. Mucocutaneous criteria for the diagnosis of Behçet's disease: an analysis of clinicopathologic data from multiple international centers. J Am Acad Dermatol. 1995. 32:968–976.
Article8. Takeno M, Kariyone A, Yamashita N, Takiguchi M, Mizushima Y, Kaneoka H, et al. Excessive function of peripheral blood neutrophils from patients with Behçet's disease and from HLA-B51 transgenic mice. Arthritis Rheum. 1995. 38:426–433.
Article9. Sakane T. New perspective on Behçet's disease. Int Rev Immunol. 1997. 14:89–96.
Article10. Sahin S, Akoğlu T, Direskeneli H, Sen LS, Lawrence R. Neutrophil adhesion to endothelial cells and factors affecting adhesion in patients with Behçet's disease. Ann Rheum Dis. 1996. 55:128–133.
Article11. Rizzi R, Bruno S, Dammacco R. Behçet's disease: an immune-mediated vasculitis involving vessels of all sizes. Int J Clin Lab Res. 1997. 27:225–232.
Article12. Zouboulis CC, May T. Pathogenesis of Adamantiades-Behçet's disease. Med Microbiol Immunol. 2003. 192:149–155.
Article13. Pietzsch J, Hoppmann S. Human S100A12: a novel key player in inflammation? Amino Acids. 2009. 36:381–389.
Article14. Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001. 69:986–994.15. Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999. 97:889–901.16. Foell D, Wittkowski H, Hammerschmidt I, Wulffraat N, Schmeling H, Frosch M, et al. Monitoring neutrophil activation in juvenile rheumatoid arthritis by S100A12 serum concentrations. Arthritis Rheum. 2004. 50:1286–1295.
Article17. Boussac M, Garin J. Calcium-dependent secretion in human neutrophils: a proteomic approach. Electrophoresis. 2000. 21:665–672.
Article18. Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004. 50:3762–3771.
Article19. Ye F, Foell D, Hirono KI, Vogl T, Rui C, Yu X, et al. Neutrophil-derived S100A12 is profoundly upregulated in the early stage of acute Kawasaki disease. Am J Cardiol. 2004. 94:840–844.
Article20. Foell D, Ichida F, Vogl T, Yu X, Chen R, Miyawaki T, et al. S100A12 (EN-RAGE) in monitoring Kawasaki disease. Lancet. 2003. 361:1270–1272.
Article21. Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003. 52:847–853.
Article22. Foell D, Hernández-Rodríguez J, Sánchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol. 2004. 204:311–316.
Article23. Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004. 344:37–51.
Article24. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001. 108:949–955.
Article25. Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002. 3:123–135.
Article26. International Study Group for Behçet's disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990. 335:1078–1080.27. Freeman AF, Shulman ST. Kawasaki disease: summary of the American Heart Association guidelines. Am Fam Physician. 2006. 74:1141–1148.28. Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000. 165:5814–5821.
Article29. Leung BP, Culshaw S, Gracie JA, Hunter D, Canetti CA, Campbell C, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001. 167:2879–2886.
Article30. Freire Ade L, Bertolo MB, de Pinho AJ Jr, Samara AM, Fernandes SR. Increased serum levels of interleukin-8 in polyarteritis nodosa and Behçet's disease. Clin Rheumatol. 2004. 23:203–205.
Article31. Gür-Toy G, Lenk N, Yalcin B, Aksaray S, Alli N. Serum interleukin-8 as a serologic marker of activity in Behçet's disease. Int J Dermatol. 2005. 44:657–660.32. Durmazlar SP, Ulkar GB, Eskioglu F, Tatlican S, Mert A, Akgul A. Significance of serum interleukin-8 levels in patients with Behçet's disease: high levels may indicate vascular involvement. Int J Dermatol. 2009. 48:259–264.33. Todaro M, Zerilli M, Triolo G, Iovino F, Patti M, Accardo-Palumbo A, et al. NF-κB protects Behçet's disease T cells against CD95-induced apoptosis up-regulating antiapoptotic proteins. Arthritis Rheum. 2005. 52:2179–2191.
Article34. Evereklioglu C, Er H, Türköz Y, Cekmen M. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet's disease. Mediators Inflamm. 2002. 11:87–93.
Article35. Oztas MO, Onder M, Gurer MA, Bukan N, Sancak B. Serum interleukin 18 and tumour necrosis factor-alpha levels are increased in Behçet's disease. Clin Exp Dermatol. 2005. 30:61–63.
Article36. Raziuddin S, al-Dalaan A, Bahabri S, Siraj AK, al-Sedairy S. Divergent cytokine production profile in Behçet's disease. Altered Th1/Th2 cell cytokine pattern. J Rheumatol. 1998. 25:329–333.37. Akdeniz N, Esrefoglu M, Keleş MS, Karakuzu A, Atasoy M. Serum interleukin-2, interleukin-6, tumour necrosis factor-alpha and nitric oxide levels in patients with Behcet's disease. Ann Acad Med Singapore. 2004. 33:596–599.38. Kose O, Stewart J, Waseem A, Lalli A, Fortune F. Expression of cytokeratins, adhesion and activation molecules in oral ulcers of Behçet's disease. Clin Exp Dermatol. 2008. 33:62–69.
Article39. Ahn SK, Choi EH, Lee SH, Lee S, Lee WS, Bong JP. Immunohistochemical study of Behcet's disease comparision of erythema nodosum-like lesion of Behcet s disease and erythema nodosum. J Wonju Coll Med. 1993. 6:214–223.40. Ozoran K, Aydintuğ O, Tokgöz G, Düzgün N, Tutkak H, Gürler A. Serum levels of interleukin-8 in patients with Behçet's disease. Ann Rheum Dis. 1995. 54:610.41. Zouboulis CC, Katsantonis J, Ketteler R, Treudler R, Kaklamani E, Hornemann S, et al. Adamantiades-Behçet's disease: interleukin-8 is increased in serum of patients with active oral and neurological manifestations and is secreted by small vessel endothelial cells. Arch Dermatol Res. 2000. 292:279–284.
Article42. Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992. 121:924–926.
Article43. Suzuki H, Noda E, Miyawaki M, Takeuchi T, Uemura S, Koike M. Serum levels of neutrophil activation cytokines in Kawasaki disease. Pediatr Int. 2001. 43:115–119.
Article44. Asano T, Ogawa S. Expression of IL-8 in Kawasaki disease. Clin Exp Immunol. 2000. 122:514–519.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associations between Soluble Receptor for Advanced Glycation End Products (sRAGE) and S100A12 (EN-RAGE) with Mortality in Long-term Hemodialysis Patients
- A Case of Behcet's Disease Associated with Protein S Deficiency
- Fecal S100A12 is associated with future hospitalization and step-up of medical treatment in patients with Crohn’s disease in clinical remission: a pilot study
- Decreased Protein S Activity Related to the Disease Activity of Behcet's Disease
- Extracellular High-Mobility Group Box 1 is Increased in Patients with Behcet's Disease with Intestinal Involvement