Ann Dermatol.
2014 Apr;26(2):203-208. 10.5021/ad.2014.26.2.203.
Immunohistochemical Analysis of Interleukin-17 Producing T Helper Cells and Regulatory T Cells Infiltration in Annular Erythema Associated with Sjogren's Syndrome
- Affiliations
-
- 1Department of Dermatology Integrated Medicine, Graduate School of Medicine, Osaka University, Osaka, Japan. tanemura@derma.med.osaka-u.ac.jp
- 2Department of Dermatology, Kinki University Faculty of Medicine, Osaka, Japan.
- KMID: 2171656
- DOI: http://doi.org/10.5021/ad.2014.26.2.203
Abstract
- BACKGROUND
Peculiar erythema known as annular erythema associated with Sjogren's syndrome (AESS) can be differentiated from autoimmune annular erythema and subacute cutaneous lupus erythematosus, both clinically and histologically. However, there are no detailed investigations on immune competent cells infiltration.
OBJECTIVE
Preferential infiltration of interleukin-17-producing T helper (Th17) cells and regulatory T (Treg) cells into the labial salivary gland is reported to play a role in maintaining mucoepithelitis in patients with Sjogren's syndrome. In this study, we evaluated Th17 and Treg cell infiltration into the lesional skin of AESS.
METHODS
We analyzed the numbers and infiltration patterns of Th17 and FoxP3 (+) Treg cells in seven cases of AESS using immunohistochemistry. Seven patients with systemic lupus erythematosus (SLE), atopic dermatitis (AD) and psoriasis vulgaris (PV), which are representatives of Th17 cell-involved skin disorders, were enrolled as disease controls.
RESULTS
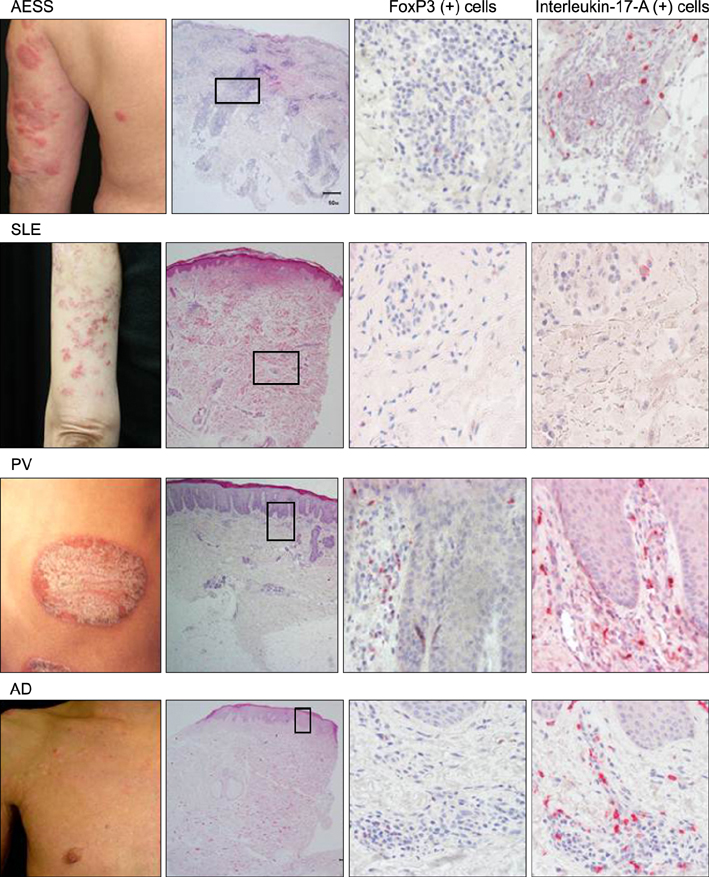
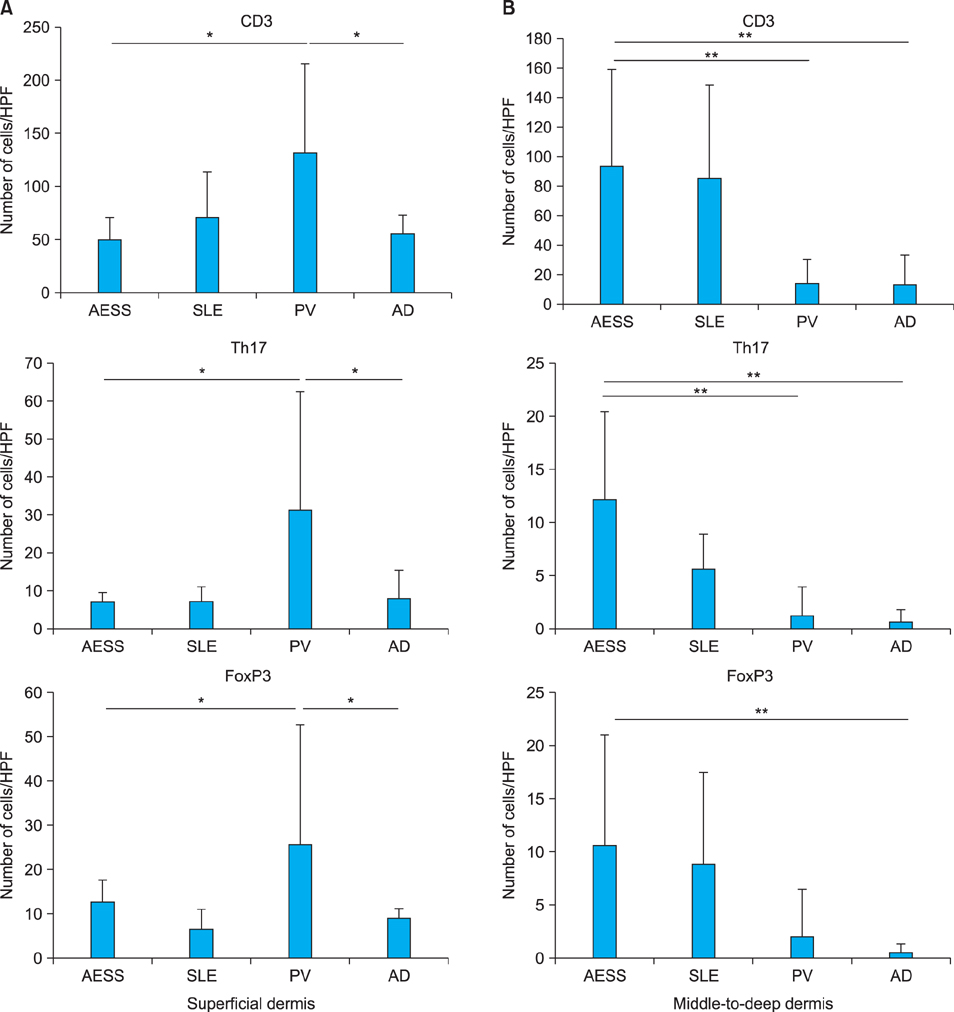
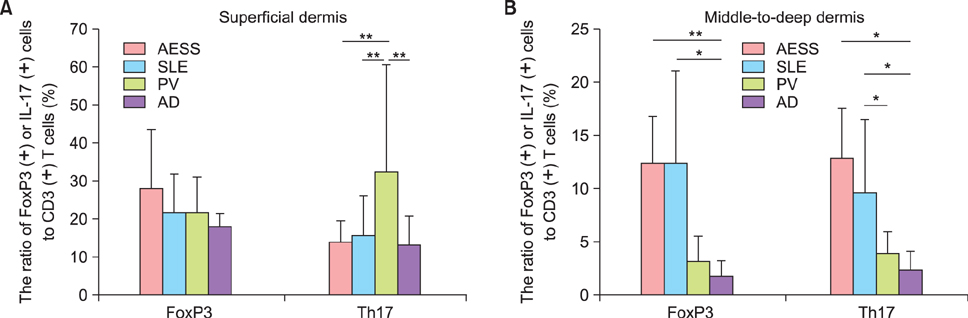
Periappendageal and epidermal changes, such as follicular plugging and liquefaction, were evident in the annular erythema of SLE, not AESS, tissue samples. In AESS tissue samples, dense perivascular and periappendageal infiltration of lymph cells was observed in the middle-to-deep dermis, as previously described, in contrast to the superficial infiltration pattern observed in both AD and PV samples. While the total number of infiltrated lymphocytes was similar between AESS and SLE tissue samples, Th17 cells were found to be preferentially infiltrated in the middle-to-deep dermis in AESS samples.
CONCLUSION
These results suggest that an increased number and distribution of infiltration of Th17 cells is a preferential feature of AESS, rather than a characteristic feature of annular erythema of SLE.
Keyword
MeSH Terms
Figure
Reference
-
1. Teramoto N, Katayama I, Arai H, Eto H, Kamimura K, Uetsuka M, et al. Annular erythema: a possible association with primary Sjögren's syndrome. J Am Acad Dermatol. 1989; 20:596–601.
Article2. Katayama I, Teramoto N, Arai H, Nishioka K, Nishiyama S. Annular erythema. A comparative study of Sjögren syndrome with subacute cutaneous lupus erythematosus. Int J Dermatol. 1991; 30:635–639.3. Katayama I, Yamamoto T, Otoyama K, Matsunaga T, Nishioka K. Clinical and immunological analysis of annular erythema associated with Sjögren syndrome. Dermatology. 1994; 189:Suppl 1. 14–17.
Article4. Katayama I, Kotobuki Y, Kiyohara E, Murota H. Annular erythema associated with Sjögren's syndrome: review of the literature on the management and clinical analysis of skin lesions. Mod Rheumatol. 2010; 20:123–129.
Article5. Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren's syndrome: findings in humans and mice. Arthritis Rheum. 2008; 58:734–743.
Article6. Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008; 181:2898–2906.
Article7. Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am J Pathol. 2009; 175:1167–1177.
Article8. Youinou P, Pers JO. Disturbance of cytokine networks in Sjögren's syndrome. Arthritis Res Ther. 2011; 13:227.
Article9. Szodoray P, Papp G, Horvath IF, Barath S, Sipka S, Nakken B, et al. Cells with regulatory function of the innate and adaptive immune system in primary Sjögren's syndrome. Clin Exp Immunol. 2009; 157:343–349.
Article10. Katayama I, Kohno Y, Akiyama K, Ikezawa Z, Kondo N, Tamaki K, et al. Japanese Society of Allergology. Japanese guideline for atopic dermatitis. Allergol Int. 2011; 60:205–220.
Article11. Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren's syndrome (1999): availability and validity. Mod Rheumatol. 2004; 14:425–434.
Article12. Watanabe T, Tsuchida T. Classification of lupus erythematosus based upon cutaneous manifestations. Dermatological, systemic and laboratory findings in 191 patients. Dermatology. 1995; 190:277–283.
Article13. Katayama I, Asai T, Nishioka K, Nishiyama S. Annular erythema associated with primary Sjögren syndrome: analysis of T cell subsets in cutaneous infiltrates. J Am Acad Dermatol. 1989; 21:1218–1221.
Article14. Yamamoto T, Katayama I, Nishioka K. Analysis of T cell receptor Vbeta repertoires of annular erythema associated with Sjögren's syndrome. Eur J Dermatol. 1998; 8:248–251.15. Arakaki R, Ishimaru N, Saito I, Kobayashi M, Yasui N, Sumida T, et al. Development of autoimmune exocrinopathy resembling Sjögren's syndrome in adoptively transferred mice with autoreactive CD4+ T cells. Arthritis Rheum. 2003; 48:3603–3609.
Article16. Shiari R, Kobayashi I, Toita N, Hatano N, Kawamura N, Okano M, et al. Epitope mapping of anti-alpha-fodrin autoantibody in juvenile Sjögren's syndrome: difference in major epitopes between primary and secondary cases. J Rheumatol. 2006; 33:1395–1400.17. Sumida T, Tsuboi H, Iizuka M, Nakamura Y, Matsumoto I. Functional role of M3 muscarinic acetylcholine receptor (M3R) reactive T cells and anti-M3R autoantibodies in patients with Sjögren's syndrome. Autoimmun Rev. 2010; 9:615–617.
Article18. Miyagawa S, Dohi K, Shima H, Shirai T. HLA antigens in anti-Ro(SS-A)-positive patients with recurrent annular erythema. J Am Acad Dermatol. 1993; 28:185–188.
Article19. Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008; 128:1207–1211.
Article20. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008; 118:597–607.
Article21. Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008; 128:2625–2630.
Article22. McCauliffe DP, Faircloth E, Wang L, Hashimoto T, Hoshino Y, Nishikawa T. Similar Ro/SS-A autoantibody epitope and titer responses in annular erythema of Sjögren's syndrome and subacute cutaneous lupus erythematosus. Arch Dermatol. 1996; 132:528–531.
Article23. Ruzicka T, Faes J, Bergner T, Peter RU, Braun-Falco O. Annular erythema associated with Sjögren's syndrome: a variant of systemic lupus erythematosus. J Am Acad Dermatol. 1991; 25:557–560.
Article24. Provost TT, Talal N, Harley JB, Reichlin M, Alexander E. The relationship between anti-Ro (SS-A) antibody-positive Sjögren's syndrome and anti-Ro (SS-A) antibody-positive lupus erythematosus. Arch Dermatol. 1988; 124:63–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Recurrent Annular Erythema of Sjogren's Syndrome Treated with Hydroxychloroquine
- Primary Sjogren's Syndrome Manifested asAnnular Erythema and Fever of an Unknown Origin
- Annular Erythema Associated with Anti-Ro/La Antibody Occurring in Plantar Area
- IL-17A-Producing Foxp3⺠Regulatory T Cells and Human Diseases
- Primary Sjogren's syndrome manifested as multiple sclerosis and cutaneous erythematous lesions: a case report