Ann Dermatol.
2014 Apr;26(2):195-202. 10.5021/ad.2014.26.2.195.
Prognostic and Clinicopathologic Associations of BRAF Mutation in Primary Acral Lentiginous Melanoma in Korean Patients: A Preliminary Study
- Affiliations
-
- 1Department of Dermatology, Dong-A University College of Medicine, Busan, Korea. khsong@dau.ac.kr
- 2Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Pathology, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2171655
- DOI: http://doi.org/10.5021/ad.2014.26.2.195
Abstract
- BACKGROUND
In the majority of melanomas, the RAS/RAF/MEK/ERK signaling pathway is constitutively activated, due to oncogenic mutations in the BRAF and NRAS genes. The BRAF mutation has been mainly described in Caucasian melanomas. However, there is a lack of study evaluating the status, and the clinical significance, of BRAF mutation in the Asian population.
OBJECTIVE
This study was aimed to determine the frequency of BRAF mutation, and to evaluate the correlation of BRAF status with clinicopathologic features and outcomes, in Korean primary acral lentiginous melanoma (ALM) patients.
METHODS
ALM samples (n=36) were analyzed for the BRAF V600E mutation, by dual-priming oligonucleotide (DPO) based real-time polymerase chain reaction. The clinicopathologic features and prognosis of the patients were analyzed with BRAF mutation status.
RESULTS
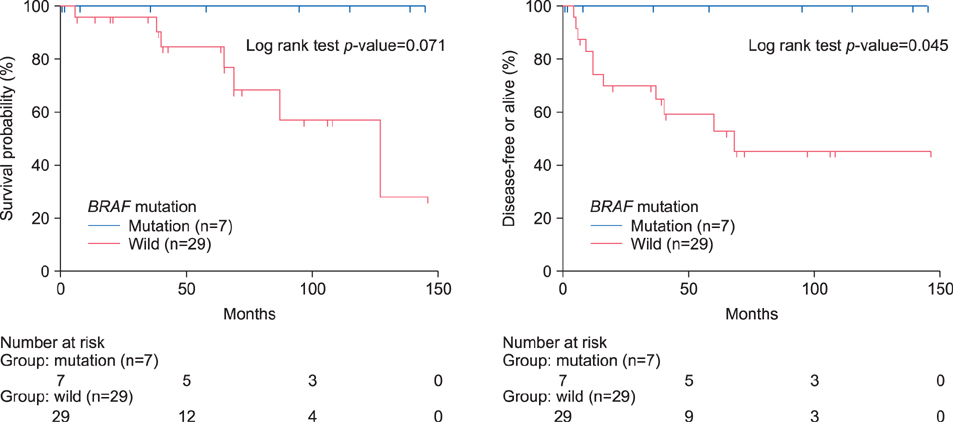
The incidence of BRAF V600E mutation was 19.4% (7/36). The BRAF V600E mutations were not associated with clinicopathologic features, except for the age factor. All of the BRAF-mutant patients survived without recurrence or metastasis, and have a better clinical outcome than BRAF wild-type patients.
CONCLUSION
In Korean primary ALM, a low frequency of BRAF mutation was shown; and BRAF mutation presented with a favorable prognosis. These results indicate that other distinctive genetic mechanisms may have more important roles in the development and progression of disease. Further multicenter study with large sample size is firmly needed, to confirm the results of our preliminary study.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Amelanotic Acral Melanoma Associated with KIT Mutation and Vitiligo
Young Jee Kim, Jee-Bum Lee, Seong-Jin Kim, Seung-Chul Lee, Young Ho Won, Sook Jung Yun
Ann Dermatol. 2015;27(2):201-205. doi: 10.5021/ad.2015.27.2.201.
Reference
-
1. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417:949–954.
Article2. Edlundh-Rose E, Egyházi S, Omholt K, Månsson-Brahme E, Platz A, Hansson J, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006; 16:471–478.
Article3. Saldanha G, Potter L, Daforno P, Pringle JH. Cutaneous melanoma subtypes show different BRAF and NRAS mutation frequencies. Clin Cancer Res. 2006; 12:4499–4505.
Article4. Lang J, MacKie RM. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. J Invest Dermatol. 2005; 125:575–579.
Article5. Devitt B, Liu W, Salemi R, Wolfe R, Kelly J, Tzen CY, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res. 2011; 24:666–672.
Article6. Akslen LA, Angelini S, Straume O, Bachmann IM, Molven A, Hemminki K, et al. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J Invest Dermatol. 2005; 125:312–317.
Article7. Kumar R, Angelini S, Czene K, Sauroja I, Hahka-Kemppinen M, Pyrhönen S, et al. BRAF mutations in metastatic melanoma: a possible association with clinical outcome. Clin Cancer Res. 2003; 9:3362–3368.8. Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011; 29:1239–1246.
Article9. Liu W, Kelly JW, Trivett M, Murray WK, Dowling JP, Wolfe R, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007; 127:900–905.
Article10. Si L, Kong Y, Xu X, Flaherty KT, Sheng X, Cui C, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012; 48:94–100.
Article11. Qi RQ, He L, Zheng S, Hong Y, Ma L, Zhang S, et al. BRAF exon 15 T1799A mutation is common in melanocytic nevi, but less prevalent in cutaneous malignant melanoma, in Chinese Han. J Invest Dermatol. 2011; 131:1129–1138.
Article12. Zhou QM, Li W, Zhang X, Chen YB, Chen XC, Guan YX, et al. The mutation profiles of common oncogenes involved in melanoma in southern China. J Invest Dermatol. 2012; 132:1935–1937.
Article13. Jin SA, Chun SM, Choi YD, Kweon SS, Jung ST, Shim HJ, et al. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J Invest Dermatol. 2013; 133:579–582.
Article14. Ashida A, Uhara H, Kiniwa Y, Oguchi M, Murata H, Goto Y, et al. Assessment of BRAF and KIT mutations in Japanese melanoma patients. J Dermatol Sci. 2012; 66:240–242.
Article15. Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009; 27:6199–6206.
Article16. Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, et al. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin Cancer Res. 2002; 8:3728–3733.17. Peyssonnaux C, Eychène A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001; 93:53–62.
Article18. Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003; 33:19–20.
Article19. Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005; 436:720–724.
Article20. Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer. 2006; 95:496–505.
Article21. Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005; 353:2135–2147.
Article22. Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am J Pathol. 2003; 163:1765–1770.
Article23. Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003; 95:1878–1890.
Article24. Benlloch S, Payá A, Alenda C, Bessa X, Andreu M, Jover R, et al. Detection of BRAF V600E mutation in colorectal cancer: comparison of automatic sequencing and real-time chemistry methodology. J Mol Diagn. 2006; 8:540–543.25. Bauer J, Büttner P, Murali R, Okamoto I, Kolaitis NA, Landi MT, et al. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res. 2011; 24:345–351.
Article26. Takata M, Goto Y, Ichii N, Yamaura M, Murata H, Koga H, et al. Constitutive activation of the mitogen-activated protein kinase signaling pathway in acral melanomas. J Invest Dermatol. 2005; 125:318–322.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sensitivity and Usefulness of VE1 Immunohistochemical Staining in Acral Melanomas with BRAF Mutation
- A Case of Thin Acral Lentiginous Melanoma with Lymph Node Metastasis and Regression
- Acral Lentiginous Melanoma Developing during Long-standing Atypical Melanosis: Usefulness of Dermoscopy for Detection of Early Acral Melanoma
- A Case of Acral Lentiginous Melanoma
- Acral Lentiginous Melanoma in situ