Ewha Med J.
2014 Mar;37(1):16-25. 10.12771/emj.2014.37.1.16.
Renal Cell Neoplasms: Recent Advances
- Affiliations
-
- 1Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea. jaero@houstonmethodist.org
- 2Department of Pathology and Genomic Medicine, The Methodist Hospital and Weill Medical College of Cornell University, Houston, TX, USA.
- 3Global Top 5 Research Program, Ewha Womans University, Seoul, Korea.
- KMID: 2171269
- DOI: http://doi.org/10.12771/emj.2014.37.1.16
Abstract
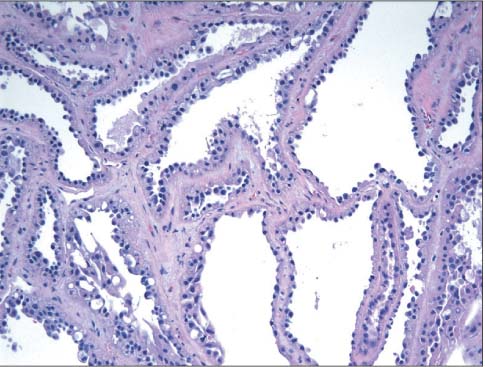
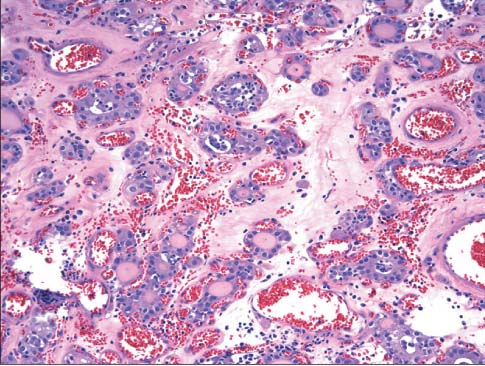
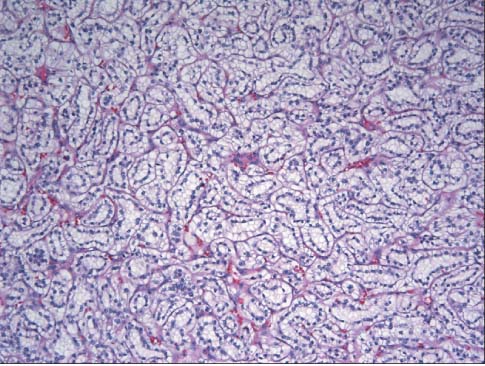
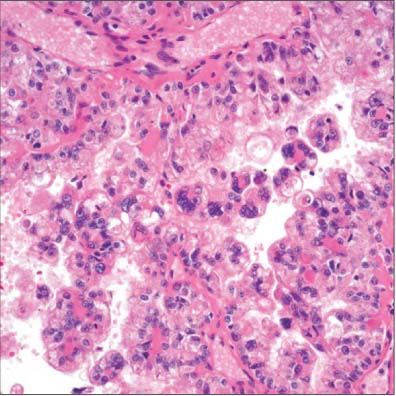
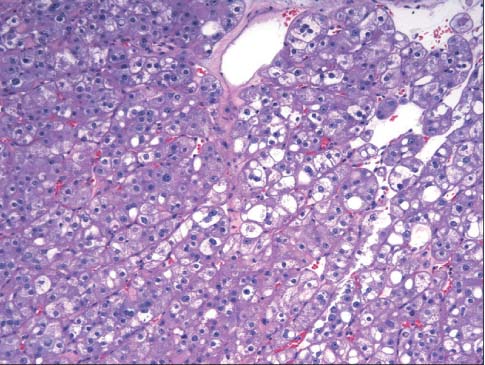
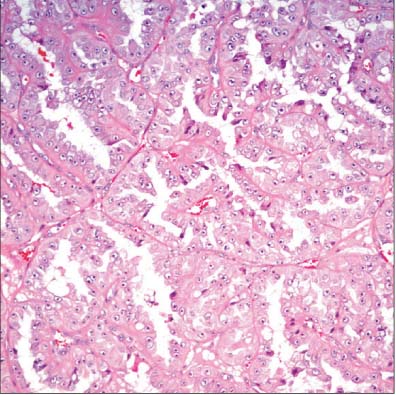
- The incidence of renal cell neoplasms has been increased in worldwide as well as in Korea. Even though the World Health Organization (WHO) Classification of renal tumors (2004) is currently used, new entities require to be added in the updated classification because of recent modification with our understanding of the molecular biology and different clinical behavior of new renal tumors. In this review, recently described tumors and candidate entities will be discussed. It is of importance to know these new entities for the proper diagnosis, treatment, and their prognosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.2. National Cancer Information Center. Available from: http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040102000000.3. Eble J, Epstein J, Sesterhenn I, Sauter G. Tumors of the urinary system and male genital organs. Lyon, France: IARC Press;2004.4. Amin MB, MacLennan GT, Gupta R, Grignon D, Paraf F, Vieillefond A, et al. Tubulocystic carcinoma of the kidney: clinicopathologic analysis of 31 cases of a distinctive rare subtype of renal cell carcinoma. Am J Surg Pathol. 2009; 33:384–392.5. Azoulay S, Vieillefond A, Paraf F, Pasquier D, Cussenot O, Callard P, et al. Tubulocystic carcinoma of the kidney: a new entity among renal tumors. Virchows Arch. 2007; 451:905–909.6. Yang XJ, Zhou M, Hes O, Shen S, Li R, Lopez J, et al. Tubulocystic carcinoma of the kidney: clinicopathologic and molecular characterization. Am J Surg Pathol. 2008; 32:177–187.7. Osunkoya AO, Young AN, Wang W, Netto GJ, Epstein JI. Comparison of gene expression profiles in tubulocystic carcinoma and collecting duct carcinoma of the kidney. Am J Surg Pathol. 2009; 33:1103–1106.8. Zhou M, Yang XJ, Lopez JI, Shah RB, Hes O, Shen SS, et al. Renal tubulocystic carcinoma is closely related to papillary renal cell carcinoma: implications for pathologic classification. Am J Surg Pathol. 2009; 33:1840–1849.9. Al-Hussain TO, Cheng L, Zhang S, Epstein JI. Tubulocystic carcinoma of the kidney with poorly differentiated foci: a series of 3 cases with fluorescence in situ hybridization analysis. Hum Pathol. 2013; 44:1406–1411.10. Jung SJ, Chung JI, Park SH, Ayala AG, Ro JY. Thyroid follicular carcinoma-like tumor of kidney: a case report with morphologic, immunohistochemical, and genetic analysis. Am J Surg Pathol. 2006; 30:411–415.11. Amin MB, Gupta R, Ondrej H, McKenney JK, Michal M, Young AN, et al. Primary thyroid-like follicular carcinoma of the kidney: report of 6 cases of a histologically distinctive adult renal epithelial neoplasm. Am J Surg Pathol. 2009; 33:393–400.12. Dhillon J, Tannir NM, Matin SF, Tamboli P, Czerniak BA, Guo CC. Thyroid-like follicular carcinoma of the kidney with metastases to the lungs and retroperitoneal lymph nodes. Hum Pathol. 2011; 42:146–150.13. Khoja HA, Almutawa A, Binmahfooz A, Aslam M, Ghazi AA, Al-maiman S. Papillary thyroid carcinoma-like tumor of the kidney: a case report. Int J Surg Pathol. 2012; 20:411–415.14. Malde S, Sheikh I, Woodman I, Fish D, Bilagi P, Sheriff MK. Primary thyroid-like follicular renal cell carcinoma: an emerging entity. Case Rep Pathol. 2013; 2013:687427.15. Angell SK, Pruthi R, Freiha FS. Primary thyroidlike carcinoma of the kidney. Urology. 1996; 48:632–635.16. Sterlacci W, Verdorfer I, Gabriel M, Mikuz G. Thyroid follicular carcinoma-like renal tumor: a case report with morphologic, immunophenotypic, cytogenetic, and scintigraphic studies. Virchows Arch. 2008; 452:91–95.17. Choyke PL. Acquired cystic kidney disease. Eur Radiol. 2000; 10:1716–1721.18. Denton MD, Magee CC, Ovuworie C, Mauiyyedi S, Pascual M, Colvin RB, et al. Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: a pathologic analysis. Kidney Int. 2002; 61:2201–2209.19. Doublet JD, Peraldi MN, Gattegno B, Thibault P, Sraer JD. Renal cell carcinoma of native kidneys: prospective study of 129 renal transplant patients. J Urol. 1997; 158:42–44.20. Hughson MD, Buchwald D, Fox M. Renal neoplasia and acquired cystic kidney disease in patients receiving long-term dialysis. Arch Pathol Lab Med. 1986; 110:592–601.21. Aydin H, Chen L, Cheng L, Vaziri S, He H, Ganapathi R, et al. Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol. 2010; 34:1608–1621.22. Rohan SM, Xiao Y, Liang Y, Dudas ME, Al-Ahmadie HA, Fine SW, et al. Clear-cell papillary renal cell carcinoma: molecular and immunohistochemical analysis with emphasis on the von Hippel-Lindau gene and hypoxia-inducible factor pathway-related proteins. Mod Pathol. 2011; 24:1207–1220.23. Tickoo SK, dePeralta-Venturina MN, Harik LR, Worcester HD, Salama ME, Young AN, et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol. 2006; 30:141–153.24. Sule N, Yakupoglu U, Shen SS, Krishnan B, Yang G, Lerner S, et al. Calcium oxalate deposition in renal cell carcinoma associated with acquired cystic kidney disease: a comprehensive study. Am J Surg Pathol. 2005; 29:443–451.25. Pan CC, Chen YJ, Chang LC, Chang YH, Ho DM. Immunohistochemical and molecular genetic profiling of acquired cystic disease-associated renal cell carcinoma. Histopathology. 2009; 55:145–153.26. Kuroda N, Tamura M, Taguchi T, Tominaga A, Hes O, Michal M, et al. Sarcomatoid acquired cystic disease-associated renal cell carcinoma. Histol Histopathol. 2008; 23:1327–1331.27. Gobbo S, Eble JN, Grignon DJ, Martignoni G, MacLennan GT, Shah RB, et al. Clear cell papillary renal cell carcinoma: a distinct histopathologic and molecular genetic entity. Am J Surg Pathol. 2008; 32:1239–1245.28. Adam J, Couturier J, Molinie V, Vieillefond A, Sibony M. Clear-cell papillary renal cell carcinoma: 24 cases of a distinct low-grade renal tumour and a comparative genomic hybridization array study of seven cases. Histopathology. 2011; 58:1064–1071.29. Bhatnagar R, Alexiev BA. Renal-cell carcinomas in end-stage kidneys: a clinicopathological study with emphasis on clear-cell papillary renal-cell carcinoma and acquired cystic kidney disease-associated carcinoma. Int J Surg Pathol. 2012; 20:19–28.30. Srigley JR, Delahunt B. Uncommon and recently described renal carcinomas. Mod Pathol. 2009; 22:Suppl 2. S2–S23.31. Tickoo SK, Reuter VE. Differential diagnosis of renal tumors with papillary architecture. Adv Anat Pathol. 2011; 18:120–132.32. Zhou H, Zheng S, Truong LD, Ro JY, Ayala AG, Shen SS. Clear cell papillary renal cell carcinoma is the fourth most common histologic type of renal cell carcinoma in 290 consecutive nephrectomies for renal cell carcinoma. Hum Pathol. 2014; 45:59–64.33. Enoki Y, Katoh G, Okabe H, Yanagisawa A. Clinicopathological features and CD57 expression in renal cell carcinoma in acquired cystic disease of the kidneys: with special emphasis on a relation to the duration of haemodialysis, the degree of calcium oxalate deposition, histological type, and possible tumorigenesis. Histopathology. 2010; 56:384–394.34. Argani P, Hawkins A, Griffin CA, Goldstein JD, Haas M, Beckwith JB, et al. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21.1;q12) chromosome translocation. Am J Pathol. 2001; 158:2089–2096.35. Argani P, Lae M, Hutchinson B, Reuter VE, Collins MH, Perentesis J, et al. Renal carcinomas with the t(6;11)(p21;q12): clinicopathologic features and demonstration of the specific alpha-TFEB gene fusion by immunohistochemistry, RT-PCR, and DNA PCR. Am J Surg Pathol. 2005; 29:230–240.36. Argani P, Yonescu R, Morsberger L, Morris K, Netto GJ, Smith N, et al. Molecular confirmation of t(6;11)(p21;q12) renal cell carcinoma in archival paraffin-embedded material using a break-apart TFEB FISH assay expands its clinicopathologic spectrum. Am J Surg Pathol. 2012; 36:1516–1526.37. Camparo P, Vasiliu V, Molinie V, Couturier J, Dykema KJ, Petillo D, et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am J Surg Pathol. 2008; 32:656–670.38. Inamura K, Fujiwara M, Togashi Y, Nomura K, Mukai H, Fujii Y, et al. Diverse fusion patterns and heterogeneous clinicopathologic features of renal cell carcinoma with t(6;11) translocation. Am J Surg Pathol. 2012; 36:35–42.39. Martignoni G, Pea M, Gobbo S, Brunelli M, Bonetti F, Segala D, et al. Cathepsin-K immunoreactivity distinguishes MiTF/TFE family renal translocation carcinomas from other renal carcinomas. Mod Pathol. 2009; 22:1016–1022.40. Pecciarini L, Cangi MG, Lo Cunsolo C, Macri E, Dal Cin E, Martignoni G, et al. Characterization of t(6;11)(p21;q12) in a renal-cell carcinoma of an adult patient. Genes Chromosomes Cancer. 2007; 46:419–426.41. Zhan HQ, Wang CF, Zhu XZ, Xu XL. Renal cell carcinoma with t(6;11) translocation: a patient case with a novel Alpha-TFEB fusion point. J Clin Oncol. 2010; 28:e709–e713.42. Hora M, Hes O, Urge T, Eret V, Klecka J, Michal M. A distinctive translocation carcinoma of the kidney ["rosette-like forming", t(6;11), HMB45-positive renal tumor]. Int Urol Nephrol. 2009; 41:553–557.43. Petersson F, Vanecek T, Michal M, Martignoni G, Brunelli M, Halbhuber Z, et al. A distinctive translocation carcinoma of the kidney; "rosette forming", t(6;11), HMB45-positive renal tumor: a histomorphologic, immunohistochemical, ultrastructural, and molecular genetic study of 4 cases. Hum Pathol. 2012; 43:726–736.44. Rao Q, Liu B, Cheng L, Zhu Y, Shi QL, Wu B, et al. Renal cell carcinomas with t(6;11)(p21;q12): A clinicopathologic study emphasizing unusual morphology, novel alpha-TFEB gene fusion point, immunobiomarkers, and ultrastructural features, as well as detection of the gene fusion by fluorescence in situ hybridization. Am J Surg Pathol. 2012; 36:1327–1338.45. Suarez-Vilela D, Izquierdo-Garcia F, Mendez-Alvarez JR, Miguelez-Garcia E, Dominguez-Iglesias F. Renal translocation carcinoma with expression of TFEB: presentation of a case with distinctive histological and immunohistochemical features. Int J Surg Pathol. 2011; 19:506–509.46. Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol. 2013; 37:1469–1489.47. Crumley SM, Divatia M, Truong L, Shen S, Ayala AG, Ro JY. Renal cell carcinoma: Evolving and emerging subtypes. World J Clin Cases. 2013; 1:262–275.48. Delongchamps NB, Galmiche L, Eiss D, Rouach Y, Vogt B, Timsit MO, et al. Hybrid tumour 'oncocytoma-chromophobe renal cell carcinoma' of the kidney: a report of seven sporadic cases. BJU Int. 2009; 103:1381–1384.49. Petersson F, Gatalica Z, Grossmann P, Perez Montiel MD, Alvarado Cabrero I, Bulimbasic S, et al. Sporadic hybrid oncocytic/chromophobe tumor of the kidney: a clinicopathologic, histomorphologic, immunohistochemical, ultrastructural, and molecular cytogenetic study of 14 cases. Virchows Arch. 2010; 456:355–365.50. Waldert M, Klatte T, Haitel A, Ozsoy M, Schmidbauer J, Marberger M, et al. Hybrid renal cell carcinomas containing histopathologic features of chromophobe renal cell carcinomas and oncocytomas have excellent oncologic outcomes. Eur Urol. 2010; 57:661–665.51. Adley BP, Smith ND, Nayar R, Yang XJ. Birt-Hogg-Dube syndrome: clinicopathologic findings and genetic alterations. Arch Pathol Lab Med. 2006; 130:1865–1870.52. Gobbo S, Eble JN, Delahunt B, Grignon DJ, Samaratunga H, Martignoni G, et al. Renal cell neoplasms of oncocytosis have distinct morphologic, immunohistochemical, and cytogenetic profiles. Am J Surg Pathol. 2010; 34:620–626.53. Mai KT, Dhamanaskar P, Belanger E, Stinson WA. Hybrid chromophobe renal cell neoplasm. Pathol Res Pract. 2005; 201:385–389.54. Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, et al. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002; 26:1542–1552.55. Tickoo SK, Reuter VE, Amin MB, Srigley JR, Epstein JI, Min KW, et al. Renal oncocytosis: a morphologic study of fourteen cases. Am J Surg Pathol. 1999; 23:1094–1101.56. Warfel KA, Eble JN. Renal oncocytomatosis. J Urol. 1982; 127:1179–1180.57. Klomp JA, Petillo D, Niemi NM, Dykema KJ, Chen J, Yang XJ, et al. Birt-Hogg-Dube renal tumors are genetically distinct from other renal neoplasias and are associated with up-regulation of mitochondrial gene expression. BMC Med Genomics. 2010; 3:59.58. Murakami T, Sano F, Huang Y, Komiya A, Baba M, Osada Y, et al. Identification and characterization of Birt-Hogg-Dube associated renal carcinoma. J Pathol. 2007; 211:524–531.59. Launonen V, Vierimaa O, Kiuru M, Isola J, Roth S, Pukkala E, et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001; 98:3387–3392.60. Grubb RL 3rd, Franks ME, Toro J, Middelton L, Choyke L, Fowler S, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007; 177:2074–2079. discussion 2079–discussion 2080.61. Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007; 31:1578–1585.62. Michal M, Hes O, Nemcova J, Sima R, Kuroda N, Bulimbasic S, et al. Renal angiomyoadenomatous tumor: morphologic, immunohistochemical, and molecular genetic study of a distinct entity. Virchows Arch. 2009; 454:89–99.63. Kuhn E, De Anda J, Manoni S, Netto G, Rosai J. Renal cell carcinoma associated with prominent angioleiomyoma-like proliferation: Report of 5 cases and review of the literature. Am J Surg Pathol. 2006; 30:1372–1381.64. Shannon BA, Cohen RJ, Segal A, Baker EG, Murch AR. Clear cell renal cell carcinoma with smooth muscle stroma. Hum Pathol. 2009; 40:425–429.65. Kuroda N, Hosokawa T, Michal M, Hes O, Sima R, Ohe C, et al. Clear cell renal cell carcinoma with focal renal angiomyoadenomatous tumor-like area. Ann Diagn Pathol. 2011; 15:202–206.66. Kuroda N, Michal M, Hes O, Taguchi T, Tominaga A, Mizobuchi K, et al. Renal angiomyoadenomatous tumor: fluorescence in situ hybridization. Pathol Int. 2009; 59:689–691.67. Michal M, Hes O, Kuroda N, Kazakov DV, Hora M. Difference between RAT and clear cell papillary renal cell carcinoma/clear renal cell carcinoma. Virchows Arch. 2009; 454:719.68. Petersson F, Yan B, Huang J, Thamboo TP, Bing TK, Consigliere DT. Low-grade renal carcinoma with histologic features overlapping with renal angiomyoadenomatous tumor and featuring polysomy 7 and 17 and a mutation in the von Hippel-Lindau gene: report of a hybrid tumor and a few comments on renal angiomyoadenomatous tumor and papillary renal tumors with clear cells. Ann Diagn Pathol. 2011; 15:213–216.69. Verine J. Renal angiomyoadenomatous tumor: morphologic, immunohistochemical, and molecular genetic study of a distinct entity. Virchows Arch. 2009; 454:479–480.