Infect Chemother.
2014 Mar;46(1):1-12. 10.3947/ic.2014.46.1.1.
Biomarkers of Sepsis
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. cmcjh@catholic.ac.kr
- 2Vaccine Bio Research Institute, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2170454
- DOI: http://doi.org/10.3947/ic.2014.46.1.1
Abstract
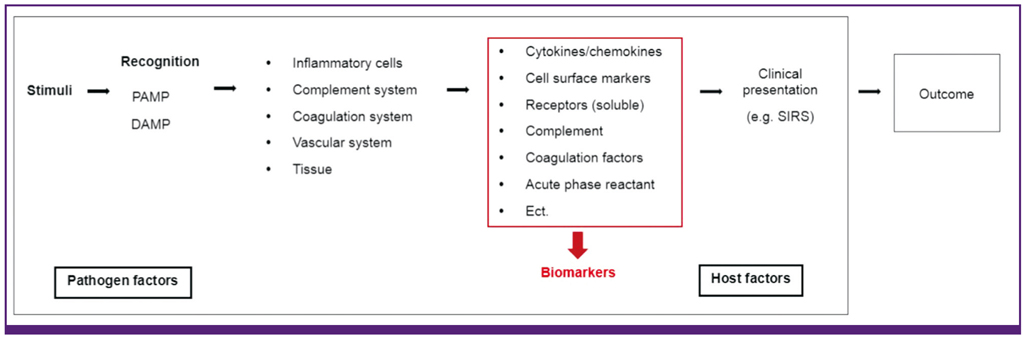
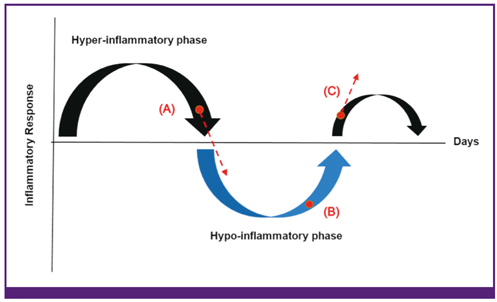
- Sepsis remains a leading cause of death in critically ill patients, despite efforts to improve patient outcome. Thus far, no magic drugs exist for severe sepsis and septic shock. Instead, early diagnosis and prompt initial management such as early goal-directed therapy are key to improve sepsis outcome. For early detection of sepsis, biological markers (biomarkers) can help clinicians to distinguish infection from host response to inflammation. Ideally, biomarkers can be used for risk stratification, diagnosis, monitoring of treatment responses, and outcome prediction. More than 170 biomarkers have been identified as useful for evaluating sepsis, including C-reactive protein, procalcitonin, various cytokines, and cell surface markers. Recently, studies have reported on the usefulness of biomarker-guided antibiotic stewardships. However, the other side of these numerous biomarkers is that no novel single laboratory marker can diagnose, predict, and track the treatment of sepsis. The purpose of this review is to summarize several key biomarkers from recent sepsis studies.
MeSH Terms
Figure
Cited by 2 articles
-
Lipidomic analysis of plasma lipids composition changes in septic mice
Won-Gyun Ahn, Jun-Sub Jung, Dong-Keun Song
Korean J Physiol Pharmacol. 2018;22(4):399-408. doi: 10.4196/kjpp.2018.22.4.399.An Update on Sepsis Biomarkers
Mi-Hee Kim, Jung-Hyun Choi
Infect Chemother. 2020;52(1):1-18. doi: 10.3947/ic.2020.52.1.1.
Reference
-
1. Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010; 36:222–231.
Article2. Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012; 366:2055–2064.
Article3. Vincent JL. The rise and fall of drotrecogin alfa (activated). Lancet Infect Dis. 2012; 12:649–651.
Article4. Guntupalli K, Dean N, Morris PE, Bandi V, Margolis B, Rivers E, Levy M, Lodato RF, Ismail PM, Reese A, Schaumberg JP, Malik R, Dellinger RP. TLF LF-0801 Investigator Group. A phase 2 randomized, double-blind, placebo-controlled study of the safety and efficacy of talactoferrin in patients with severe sepsis. Crit Care Med. 2013; 41:706–716.
Article5. McCulloh R, Opal SM. Human recombinant lactoferrin for sepsis: too good to be true? Crit Care Med. 2013; 41:908–909.6. Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D, Tidswell M, Jauregui L, Krell K, Pachl J, Takahashi T, Peckelsen C, Cordasco E, Chang CS, Oeyen S, Aikawa N, Maruyama T, Schein R, Kalil AC, Van Nuffelen M, Lynn M, Rossignol DP, Gogate J, Roberts MB, Wheeler JL, Vincent JL. ACCESS Study Group. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013; 309:1154–1162.
Article7. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41:580–637.
Article8. Choi JH. Recent evidences of sepsis treatment. Infect Chemother. 2008; 40:67–75.
Article9. Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, Black E, Schwartz JS, Moore R, Johnson BL Jr, Platt R. Academic Medical Center Consortium Sepsis Project Working Group. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997; 278:234–240.
Article10. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006; 34:344–353.
Article11. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003; 348:138–150.
Article12. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013; 13:260–268.
Article13. Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010; 184:3768–3779.
Article14. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009; 180:640–648.
Article15. Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011; 306:2594–2605.
Article16. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001; 69:89–95.17. Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010; 14:R15.
Article18. Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. Inflamm and Host Response to Injury Large Scale Collab. Res. Program. A network-based analysis of systemic inflammation in humans. Nature. 2005; 437:1032–1037.
Article19. Marshall JC, Reinhart K. International Sepsis Forum. Biomarkers of sepsis. Crit Care Med. 2009; 37:2290–2298.
Article20. Samraj RS, Zingarelli B, Wong HR. Role of biomarkers in sepsis care. Shock. 2013; 40:358–365.
Article21. Pettilä V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002; 28:1220–1225.
Article22. Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, Moldawer LL. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005; 23:488–493.23. Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993; 103:565–575.24. Qiu P, Cui X, Barochia A, Li Y, Natanson C, Eichacker PQ. The evolving experience with therapeutic TNF inhibition in sepsis: considering the potential influence of risk of death. Expert Opin Investig Drugs. 2011; 20:1555–1564.
Article25. Rice TW, Wheeler AP, Morris PE, Paz HL, Russell JA, Edens TR, Bernard GR. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit Care Med. 2006; 34:2271–2281.
Article26. Lvovschi V, Arnaud L, Parizot C, Freund Y, Juillien G, Ghillani-Dalbin P, Bouberima M, Larsen M, Riou B, Gorochov G, Hausfater P. Cytokine profiles in sepsis have limited relevance for stratifying patients in the emergency department: a prospective observational study. PLoS One. 2011; 6:e28870.
Article27. Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007; 11:R49.
Article28. Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, Gandía F, Resino S, Tamayo E, de Lejarazu RO, Bermejo-Martin JF. A combined score of pro- and anti-inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012; 57:332–336.
Article29. Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007; 297:353–363.
Article30. Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001; 98:3800–3808.
Article31. Gaïni S, Koldkjaer OG, Pedersen C, Pedersen SS. Procalcitonin, lipopolysaccharide-binding protein, interleukin-6 and C-reactive protein in community-acquired infections and sepsis: a prospective study. Crit Care. 2006; 10:R53.32. Opal SM, Scannon PJ, Vincent JL, White M, Carroll SF, Palardy JE, Parejo NA, Pribble JP, Lemke JH. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999; 180:1584–1589.
Article33. Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. Lipopolysaccharide binding protein in a surgical intensive care unit: a marker of sepsis? Crit Care Med. 2008; 36:2014–2022.
Article34. Choi JH, Shin WS. Pathogenesis of sepsis and concepts of immunotherapy. Korean J Infect Dis. 2000; 32:148–157.35. Sundén-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005; 33:564–573.
Article36. Hatada T, Wada H, Nobori T, Okabayashi K, Maruyama K, Abe Y, Uemoto S, Yamada S, Maruyama I. Plasma concentrations and importance of High Mobility Group Box protein in the prognosis of organ failure in patients with disseminated intravascular coagulation. Thromb Haemost. 2005; 94:975–979.
Article37. Gibot S, Massin F, Cravoisy A, Barraud D, Nace L, Levy B, Bollaert PE. High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 2007; 33:1347–1353.
Article38. Petrovsky N, Socha L, Silva D, Grossman AB, Metz C, Bucala R. Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol. 2003; 81:137–143.
Article39. Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Männel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000; 6:164–170.
Article40. Bozza FA, Gomes RN, Japiassú AM, Soares M, Castro-Faria-Neto HC, Bozza PT, Bozza MT. Macrophage migration inhibitory factor levels correlate with fatal outcome in sepsis. Shock. 2004; 22:309–313.
Article41. Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930; 52:561–571.
Article42. Vincent JL, Donadello K, Schmit X. Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin. 2011; 27:241–251.
Article43. Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, Ritz R. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000; 28:977–983.
Article44. Selberg O, Hecker H, Martin M, Klos A, Bautsch W, Köhl J. Discrimination of sepsis and systemic inflammatory response syndrome by determination of circulating plasma concentrations of procalcitonin, protein complement 3a, and interleukin-6. Crit Care Med. 2000; 28:2793–2798.
Article45. Suprin E, Camus C, Gacouin A, Le Tulzo Y, Lavoue S, Feuillu A, Thomas R. Procalcitonin: a valuable indicator of infection in a medical ICU? Intensive Care Med. 2000; 26:1232–1238.
Article46. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004; 39:206–217.
Article47. Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, Sabino H. C-reactive protein as a marker of infection in critically ill patients. Clin Microbiol Infect. 2005; 11:101–108.
Article48. Schmit X, Vincent JL. The time course of blood C-reactive protein concentrations in relation to the response to initial antimicrobial therapy in patients with sepsis. Infection. 2008; 36:213–219.
Article49. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993; 341:515–518.
Article50. O'Grady NP1, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H. American College of Critical Care Medicine. Infectious Diseases Society of America. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008; 36:1330–1349.51. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013; 13:426–435.
Article52. Reinhart K, Meisner M. Biomarkers in the critically ill patient: procalcitonin. Crit Care Clin. 2011; 27:253–263.
Article53. Schuetz P, Affolter B, Hunziker S, Winterhalder C, Fischer M, Balestra GM, Hunziker P, Marsch S. Serum procalcitonin, C-reactive protein and white blood cell levels following hypothermia after cardiac arrest: a retrospective cohort study. Eur J Clin Invest. 2010; 40:376–381.
Article54. Kopterides P, Siempos II, Tsangaris I, Tsantes A, Armaganidis A. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2010; 38:2229–2241.
Article55. Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009; 37:1670–1677.
Article56. Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012; 38:4–10.
Article57. Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005; 45:524–528.
Article58. Kruse O, Grunnet N, Barfod C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: a systematic review. Scand J Trauma Resusc Emerg Med. 2011; 19:74.
Article59. Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care. 2009; 13:R90.
Article60. Claridge JA, Crabtree TD, Pelletier SJ, Butler K, Sawyer RG, Young JS. Persistent occult hypoperfusion is associated with a significant increase in infection rate and mortality in major trauma patients. J Trauma. 2000; 48:8–14. discussion 14-5.
Article61. Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004; 32:1637–1642.
Article62. Linscheid P, Seboek D, Zulewski H, Keller U, Müller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005; 146:2699–2708.
Article63. Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000; 21:138–167.
Article64. Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Müller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005; 9:R816–R824.65. Al Shuaibi M, Bahu RR, Chaftari AM, Al Wohoush I, Shomali W, Jiang Y, Debiane L, Raad S, Jabbour J, Al Akhrass F, Hachem RY, Raad I. Pro-adrenomedullin as a novel biomarker for predicting infections and response to antimicrobials in febrile patients with hematologic malignancies. Clin Infect Dis. 2013; 56:943–950.
Article66. Suberviola B, Castellanos-Ortega A, Ruiz Ruiz A, Lopez-Hoyos M, Santibañez M. Hospital mortality prognostication in sepsis using the new biomarkers suPAR and proADM in a single determination on ICU admission. Intensive Care Med. 2013; 39:1945–1952.
Article67. Icardi M, Erickson Y, Kilborn S, Stewart B, Grief B, Scharnweber G. CD64 index provides simple and predictive testing for detection and monitoring of sepsis and bacterial infection in hospital patients. J Clin Microbiol. 2009; 47:3914–3919.
Article68. Cid J, García-Pardo G, Aguinaco R, Sánchez R, Llorente A. Neutrophil CD64: diagnostic accuracy and prognostic value in patients presenting to the emergency department. Eur J Clin Microbiol Infect Dis. 2011; 30:845–852.
Article69. Gros A, Roussel M, Sauvadet E, Gacouin A, Marqué S, Chimot L, Lavoué S, Camus C, Fest T, Le Tulzo Y. The sensitivity of neutrophil CD64 expression as a biomarker of bacterial infection is low in critically ill patients. Intensive Care Med. 2012; 38:445–452.
Article70. Cid J, Aguinaco R, Sánchez R, García-Pardo G, Llorente A. Neutrophil CD64 expression as marker of bacterial infection: a systematic review and meta-analysis. J Infect. 2010; 60:313–319.
Article71. Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001; 410:1103–1107.
Article72. Gibot S. Clinical review: role of triggering receptor expressed on myeloid cells-1 during sepsis. Crit Care. 2005; 9:485–489.73. Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009; 21:38–46.
Article74. Gibot S, Kolopp-Sarda MN, Béné MC, Cravoisy A, Levy B, Faure GC, Bollaert PE. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004; 141:9–15.
Article75. Zhang J, She D, Feng D, Jia Y, Xie L. Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict prognosis: a prospective study. BMC Infect Dis. 2011; 11:53.
Article76. Jiyong J, Tiancha H, Wei C, Huahao S. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: a meta-analysis. Intensive Care Med. 2009; 35:587–595.
Article77. Wu Y, Wang F, Fan X, Bao R, Bo L, Li J, Deng X. Accuracy of plasma sTREM-1 for sepsis diagnosis in systemic inflammatory patients: a systematic review and meta-analysis. Crit Care. 2012; 16:R229.
Article78. Jeong SJ, Song YG, Kim CO, Kim HW, Ku NS, Han SH, Choi JY, Kim JM. Measurement of plasma sTREM-1 in patients with severe sepsis receiving early goal-directed therapy and evaluation of its usefulness. Shock. 2012; 37:574–578.
Article79. Ploug M, Rønne E, Behrendt N, Jensen AL, Blasi F, Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991; 266:1926–1933.
Article80. Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002; 3:932–943.
Article81. Koch A, Voigt S, Kruschinski C, Sanson E, Dückers H, Horn A, Yagmur E, Zimmermann H, Trautwein C, Tacke F. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011; 15:R63.
Article82. Kofoed K, Andersen O, Kronborg G, Tvede M, Petersen J, Eugen-Olsen J, Larsen K. Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections: a prospective study. Crit Care. 2007; 11:R38.
Article83. Koch A, Tacke F. Why high suPAR is not super--diagnostic, prognostic and potential pathogenic properties of a novel biomarker in the ICU. Crit Care. 2011; 15:1020.
Article84. Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012; 38:1418–1428.
Article85. Donadello K, Scolletta S, Covajes C, Vincent JL. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012; 10:2.
Article86. Haupt TH, Petersen J, Ellekilde G, Klausen HH, Thorball CW, Eugen-Olsen J, Andersen O. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: a prospective observational study. Crit Care. 2012; 16:R130.
Article87. Uusitalo-Seppälä R, Huttunen R, Tarkka M, Aittoniemi J, Koskinen P, Leino A, Vahlberg T, Rintala EM. Soluble urokinase-type plasminogen activator receptor in patients with suspected infection in the emergency room: a prospective cohort study. J Intern Med. 2012; 272:247–256.
Article88. David S, Kümpers P, van Slyke P, Parikh SM. Mending leaky blood vessels: the angiopoietin-Tie2 pathway in sepsis. J Pharmacol Exp Ther. 2013; 345:2–6.
Article89. Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006; 12:235–239.
Article90. Ricciuto DR, dos Santos CC, Hawkes M, Toltl LJ, Conroy AL, Rajwans N, Lafferty EI, Cook DJ, Fox-Robichaud A, Kahnamoui K, Kain KC, Liaw PC, Liles WC. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011; 39:702–710.
Article91. Gibot S, Béné MC, Noel R, Massin F, Guy J, Cravoisy A, Barraud D, De Carvalho Bittencourt M, Quenot JP, Bollaert PE, Faure G, Charles PE. Combination biomarkers to diagnose sepsis in the critically ill patient. Am J Respir Crit Care Med. 2012; 186:65–71.
Article92. Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, Moretti E, Nguyen HB, Gunnerson KJ, Milzman D, Gaieski DF, Goyal M, Cairns CB, Ngo L, Rivers EP. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009; 37:96–104.
Article93. Peres Bota D, Mélot C, Lopes Ferreira F, Vincent JL. Infection Probability Score (IPS): A method to help assess the probability of infection in critically ill patients. Crit Care Med. 2003; 31:2579–2584.
Article94. Kofoed K, Eugen-Olsen J, Petersen J, Larsen K, Andersen O. Predicting mortality in patients with systemic inflammatory response syndrome: an evaluation of two prognostic models, two soluble receptors, and a macrophage migration inhibitory factor. Eur J Clin Microbiol Infect Dis. 2008; 27:375–383.
Article95. Harbarth S, Holeckova K, Froidevaux C, Pittet D, Ricou B, Grau GE, Vadas L, Pugin J. Geneva Sepsis Network. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am J Respir Crit Care Med. 2001; 164:396–402.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Update on Sepsis Biomarkers
- Eosinophil count and neutrophil-to-lymphocyte count ratio as biomarkers for predicting early-onset neonatal sepsis
- Infectious diseases and biomarker use
- Difference in Protein Markers According to the Survival of Sepsis Patients using Protein Chips
- Sepsis and Acute Respiratory Distress Syndrome: Recent Update