J Bone Metab.
2015 Aug;22(3):107-112. 10.11005/jbm.2015.22.3.107.
Discrepancy between Vitamin D Total Immunoassays due to Various Cross-reactivities
- Affiliations
-
- 1Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea. ajcp@cau.ac.kr
- KMID: 2170106
- DOI: http://doi.org/10.11005/jbm.2015.22.3.107
Abstract
- BACKGROUND
The purpose of this study was to find out the cause of discrepancy between various automated immunoassays for 25-hydroxy-vitamin D (25-[OH]D).
METHODS
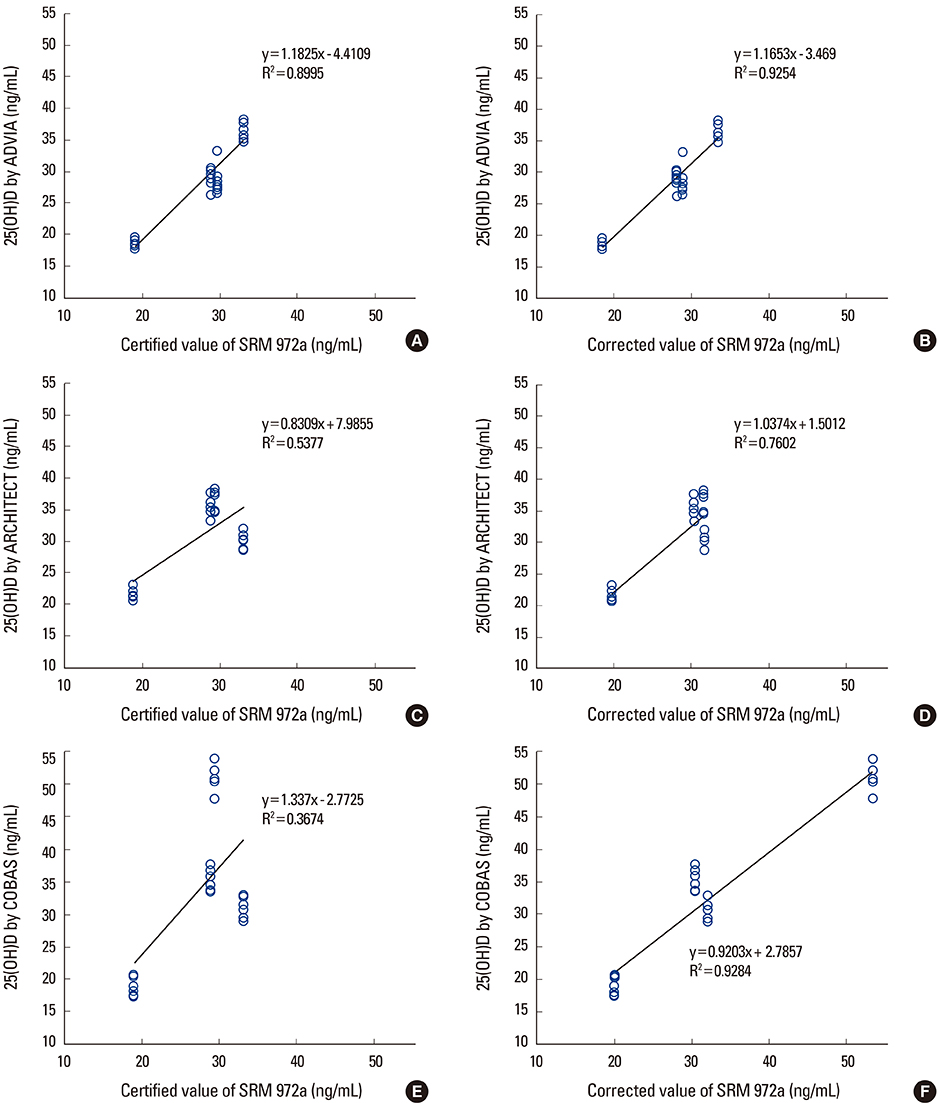
National Institute of Standards & Technology Standard Reference Material (SRM) 972a is SRM for 25-(OH)D and consists of 4 vials of frozen serum with different concentrations of 25-(OH)D. Each concentration was measured 6 times in 3 different immunoassays: ADVIA Vitamin D Total assay (Siemens Healthcare, Erlangen, Germany), ARCHITECT 25-(OH)D (Abbott Laboratories, Abbott Park, IL, USA), and COBAS Vitamin D Total assay (Roche Diagnostics, Basel, Switzerland).
RESULTS
When using the certified reference values of SRM 972a as it is, discarding the cross-reactivity of each immunoassay, for ADVIA, the coefficient of determination (R2) as a score of regression analysis was 0.8995 and maximal difference between measured value and certified reference value was 3.6 ng/mL in level 3. The R2 and maximal differences of ARCHITECT were 0.5377 and 6.9 ng/mL, respectively, in level 4. Those of COBAS were 0.3674 and 22.3 ng/mL, respectively, in level 4. When considering cross-reactivities of each immunoassays to various 25-(OH)D metabolites, the ADVIA had R2 and maximal difference of 0.9254 and 3.3 ng/mL, respectively, in level 3. For ARCHITECT, the R2 and maximal differences were 0.7602 and 5.1 ng/mL, respectively, in level 1. Those of COBAS were 0.9284 and 4.9 ng/mL, respectively, in level 1.
CONCLUSIONS
The cause of discrepancies between vitamin D immunoassays was mainly on the difference in cross-reactivities to various vitamin D metabolites. The discrepancies can be considerably decreased by considering cross-reactivities of each immunoassay.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Three Commercially Available Assays for Measurement of Vitamin D
Dahae Yang, Hyunyong Hwang
Lab Med Online. 2017;7(3):120-127. doi: 10.3343/lmo.2017.7.3.120.
Reference
-
1. Bentley J. The role of vitamin D in infants, children and young people. Nurs Child Young People. 2015; 27:28–35.
Article2. Meehan M, Penckofer S. The Role of Vitamin D in the Aging Adult. J Aging Gerontol. 2014; 2:60–71.
Article3. Garland CF, Gorham ED, Mohr SB, et al. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009; 19:468–483.
Article4. Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008; 11:7–12.
Article5. Saenger AK, Laha TJ, Bremner DE, et al. Quantification of serum 25-hydroxy-vitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006; 125:914–920.
Article6. Kwak HS, Chung HJ, Cho DH, et al. Efficacy of the measurement of 25-hydroxy-vitamin D2 and D3 levels by using PerkinElmer liquid chromatography-tandem mass spectrometry vitamin D kit compared with DiaSorin radioimmunoassay kit and Elecsys vitamin D total assay. Ann Lab Med. 2015; 35:263–265.
Article7. Farrell CJ, Martin S, McWhinney B, et al. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012; 58:531–542.
Article8. Janssen MJ, Wielders JP, Bekker CC, et al. Multicenter comparison study of current methods to measure 25-hydroxy-vitamin D in serum. Steroids. 2012; 77:1366–1372.
Article9. Gonzalez CA, Watters RL Jr. Certificate of analysis. Standard Reference Material® 972a: Vitamin D metabolites in frozen human serum. Gaithersburg, MD: National Institute of Standards & Technology;2013.10. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–281.
Article11. Zhang R, Naughton DP. Vitamin D in health and disease: current perspectives. Nutr J. 2010; 9:65.
Article12. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011; 31:48–54.
Article13. Kim MK, Baek KH, Song KH, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab. 2011; 96:3250–3256.
Article14. Carter GD. Accuracy of 25-hydroxy-vitamin D assays: confronting the issues. Curr Drug Targets. 2011; 12:19–28.
Article15. Farrell C, Soldo J, Williams P, et al. 25-Hydroxyvitamin D testing: challenging the performance of current automated immunoassays. Clin Chem Lab Med. 2012; 50:1953–1963.
Article16. Fuleihan Gel H, Bouillon R, Clarke B, et al. Serum 25-Hydroxyvitamin D Levels: Variability, Knowledge Gaps, and the Concept of a Desirable Range. J Bone Miner Res. 2015; 30:1119–1133.
Article17. Brown AJ, Ritter C, Slatopolsky E, et al. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha, 25-dihydroxy-vitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999; 73:106–113.
Article18. Fleet JC, Bradley J, Reddy GS, et al. 1 alpha,25-(OH)2-vitamin D3 analogs with minimal in vivo calcemic activity can stimulate significant transepithelial calcium transport and mRNA expression in vitro. Arch Biochem Biophys. 1996; 329:228–234.
Article19. Strathmann FG, Sadilkova K, Laha TJ, et al. 3-epi-25 hydroxy-vitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta. 2012; 413:203–206.
Article20. Singh RJ, Taylor RL, Reddy GS, et al. C-3 epimers can account for a significant proportion of total circulating 25-hydroxy-vitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006; 91:3055–3061.
Article21. Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25-hydroxy-vitamin D3 and 25-hydroxy-vitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010; 82:1942–1948.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 2 Cases of Intracranial Hemorrhae due to Late Hemorrhagic Disease of Infancy due to Vitamin K Deficiency
- Diabetes and Vitamin D
- A Case of Fatal Intracranial Hemmrhage due to Vitamin K Deficiency
- Cranial Ultrasonographic Diagnosis for A Case of intracranial Hemorrhage in Early Infancy due to Vitamin K Deficiency
- Establishment of a Korean Hepatitis B Surface Antigen Low Titer Performance Panel for Performance Validation of Hepatitis B Surface Antigen Immunoassays