Endocrinol Metab.
2014 Mar;29(1):40-47. 10.3803/EnM.2014.29.1.40.
Correlation between Expression of Glucose Transporters in Granulosa Cells and Oocyte Quality in Women with Polycystic Ovary Syndrome
- Affiliations
-
- 1Department of Biomedical Science, CHA University, Seoul, Korea. leeka@ovary.co.kr
- 2Fertility Center, CHA Gangnam Medical Center, CHA University, Seoul, Korea.
- 3Department of Applied Bioscience, CHA University, Seoul, Korea.
- KMID: 2169350
- DOI: http://doi.org/10.3803/EnM.2014.29.1.40
Abstract
- BACKGROUND
The glucose transporters (GLUTs) exhibit different tissue-specific expression. This study aimed to investigate the types of GLUTs expressed in human granulosa cells (GCs) obtained from women with polycystic ovary syndrome (PCOS) and their relationship with insulin resistance (IR) and the outcomes of in vitro maturation (IVM) of immature oocytes.
METHODS
Expression of GLUTs was evaluated in GCs from women with PCOS with or without IR. Thirty-six women with PCOS undergoing an IVM program were included. Differential gene expression between the insulin sensitive (IS) and IR group was measured by reverse transcription polymerase chain reaction.
RESULTS
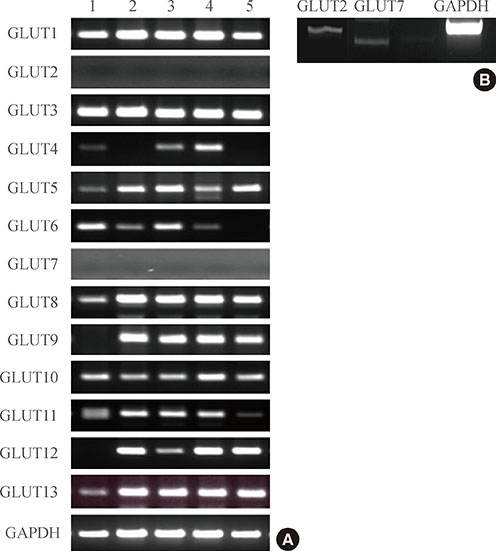
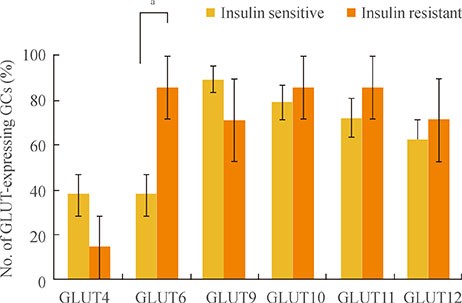
Expression of GLUTs 1, 3, 5, 8, and 13 was constitutive, whereas expression of GLUTs 2 and 7 was not observed in human GCs. The remaining GLUTs, 4, 6, 9, 10, 11, and 12, were differentially expressed among patients according to metabolic status, such as insulin sensitivity. A higher number of GCs from patients with IR (92%) expressed GLUT6 than GCs from IS PCOS patients (46.3%). Logistic regression showed that expression of GLUTs 9, 11, and 12 correlates with rates of IVM at 48 hours, fertilization, and implantation, respectively.
CONCLUSION
This is the first report describing the expression pattern of all 13 members of the GLUT family in human GCs. Results of the present study suggest that patients' insulin sensitivity regulates GLUT expression in GCs in PCOS patients, and this may control oocyte quality for IVM and subsequent processes such as fertilization and implantation in patients taking part in an in vitro fertilization program.
Keyword
MeSH Terms
-
Female
Fertilization
Fertilization in Vitro
Gene Expression
Glucose Transport Proteins, Facilitative
Glucose*
Granulosa Cells*
Humans
Insulin
Insulin Resistance
Logistic Models
Oocytes*
Polycystic Ovary Syndrome*
Polymerase Chain Reaction
Reverse Transcription
Glucose
Glucose Transport Proteins, Facilitative
Insulin
Figure
Reference
-
1. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997; 18:774–800.2. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998; 83:3078–3082.3. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004; 89:2745–2749.4. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004; 81:19–25.5. Nestler JE, Usiskin KS, Barlascini CO, Welty DF, Clore JN, Blackard WG. Suppression of serum dehydroepiandrosterone sulfate levels by insulin: an evaluation of possible mechanisms. J Clin Endocrinol Metab. 1989; 69:1040–1046.6. Dunaif A. Drug insight: insulin-sensitizing drugs in the treatment of polycystic ovary syndrome: a reappraisal. Nat Clin Pract Endocrinol Metab. 2008; 4:272–283.7. Book CB, Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1999; 84:3110–3116.8. Corbould A, Zhao H, Mirzoeva S, Aird F, Dunaif A. Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes. 2006; 55:751–759.9. Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992; 41:1257–1266.10. Wu XK, Zhou SY, Liu JX, Pollanen P, Sallinen K, Makinen M, Erkkola R. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil Steril. 2003; 80:954–965.11. Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab. 2010; 298:E141–E145.12. Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol. 2001; 18:247–256.13. Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN J Parenter Enteral Nutr. 2004; 28:364–371.14. Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004; 447:480–489.15. Wood IS, Hunter L, Trayhurn P. Expression of Class III facilitative glucose transporter genes (GLUT-10 and GLUT-12) in mouse and human adipose tissues. Biochem Biophys Res Commun. 2003; 308:43–49.16. Schurmann A. Insight into the "odd" hexose transporters GLUT3, GLUT5, and GLUT7. Am J Physiol Endocrinol Metab. 2008; 295:E225–E226.17. Rosenbaum D, Haber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: decreased expression of GLUT-4 glucose transporters in adipocytes. Am J Physiol. 1993; 264(2 Pt 1):E197–E202.18. Mozzanega B, Mioni R, Granzotto M, Chiarelli S, Xamin N, Zuliani L, Sicolo N, Marchesoni D, Vettor R. Obesity reduces the expression of GLUT4 in the endometrium of normoinsulinemic women affected by the polycystic ovary syndrome. Ann N Y Acad Sci. 2004; 1034:364–374.19. Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005; 288:E1047–E1054.20. Dunaif A, Wu X, Lee A, Diamanti-Kandarakis E. Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am J Physiol Endocrinol Metab. 2001; 281:E392–E399.21. Rajkhowa M, Brett S, Cuthbertson DJ, Lipina C, Ruiz-Alcaraz AJ, Thomas GE, Logie L, Petrie JR, Sutherland C. Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem J. 2009; 418:665–671.22. Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009; 27:32–42.23. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001; 122:829–838.24. Ryu S, Sung KC, Chang Y, Lee WY, Rhee EJ. Spectrum of insulin sensitivity in the Korean population. Metabolism. 2005; 54:1644–1651.25. Soonthornpun S, Setasuban W, Thamprasit A, Chayanunnukul W, Rattarasarn C, Geater A. Novel insulin sensitivity index derived from oral glucose tolerance test. J Clin Endocrinol Metab. 2003; 88:1019–1023.26. Chang EM, Han JE, Seok HH, Lee DR, Yoon TK, Lee WS. Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin Endocrinol (Oxf). 2013; 79:93–99.27. Fedorcsak P, Raki M, Storeng R. Characterization and depletion of leukocytes from cells isolated from the pre-ovulatory ovarian follicle. Hum Reprod. 2007; 22:989–994.28. Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics. 2002; 80:553–557.29. Suganuma N, Segade F, Matsuzu K, Bowden DW. Differential expression of facilitative glucose transporters in normal and tumour kidney tissues. BJU Int. 2007; 99:1143–1149.30. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001; 414:799–806.31. Lisinski I, Schurmann A, Joost HG, Cushman SW, Al-Hasani H. Targeting of GLUT6 (formerly GLUT9) and GLUT8 in rat adipose cells. Biochem J. 2001; 358(Pt 2):517–522.32. Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000; 106:165–169.33. Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008; 40:437–442.34. Santos P, Marques A, Antunes G, Chaveiro A, Andrade M, Borba A, da Silva FM. Effects of plasma urea nitrogen levels on the bovine oocyte ability to develop after in vitro fertilization. Reprod Domest Anim. 2009; 44:783–787.35. Doege H, Bocianski A, Scheepers A, Axer H, Eckel J, Joost HG, Schurmann A. Characterization of human glucose transporter (GLUT) 11 (encoded by SLC2A11), a novel sugar-transport facilitator specifically expressed in heart and skeletal muscle. Biochem J. 2001; 359(Pt 2):443–449.36. Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab. 2002; 282:E733–E738.37. Zhou Y, Kaye PL, Pantaleon M. Identification of the facilitative glucose transporter 12 gene Glut12 in mouse preimplantation embryos. Gene Expr Patterns. 2004; 4:621–631.38. Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. Glucose transporter GLUT12-functional characterization in Xenopus laevis oocytes. Biochem Biophys Res Commun. 2003; 308:422–426.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polycystic Ovary Syndrome in Korean Women: Clinical Characteristics and Diagnostic Criteria
- Expression of the genes for peroxisome proliferator-activated receptor-γ, cyclooxygenase-2, and proinflammatory cytokines in granulosa cells from women with polycystic ovary syndrome
- Validity and Reliability of a Korean version of Polycystic Ovary Syndrome Questionnaire
- Inter-ovarian differences in ultrasound markers of ovarian size in women with polycystic ovary syndrome
- Studies on Fibrinolytic System Behavior in Women with Polycystic Ovary Syndrome