Anat Cell Biol.
2010 Mar;43(1):36-43. 10.5115/acb.2010.43.1.36.
Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation
- Affiliations
-
- 1Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea. asch@chonbuk.ac.kr
- 2Department of Anatomy and Histology and Embryology, Yanbian University School of Medical Sciences, Jilin, China.
- 3Institute for Medical Sciences, Chonbuk National University, Jeonju, Korea.
- KMID: 2168895
- DOI: http://doi.org/10.5115/acb.2010.43.1.36
Abstract
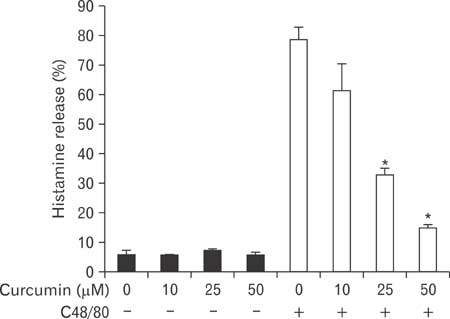
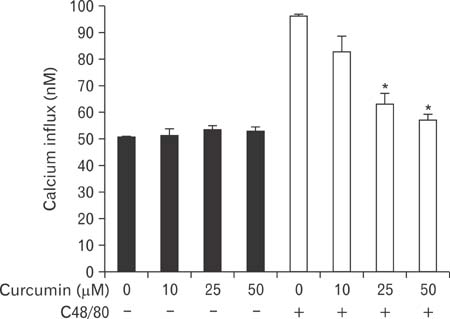
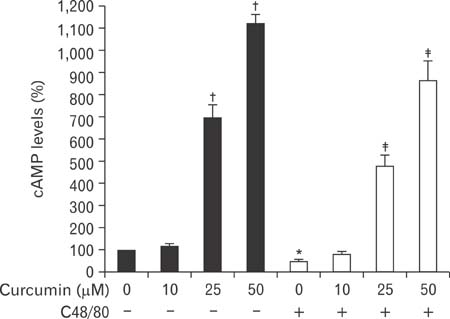
- Mast cells participate in allergies and inflammation by secreting a variety of pro-inflammatory mediators. Curcumin, the active component of turmeric, is a polyphenolic phytochemical with anti-tumor, anti-inflammatory, anti-oxidative, and anti-allergic properties. The effects of curcumin on compound 48/80-induced mast cell activation and passive cutaneous anaphylactoid reactions are unknown. In this report, we investigated the influences of curcumin on the passive cutaneous anaphylactoid response in vivo and compound 48/80-induced mast cell activation in vitro. The mechanism of action was examined by calcium uptake measurements and cAMP assays in mast cells. Curcumin significantly attenuated the mast cell-mediated passive cutaneous anaphylactoid reaction in an animal model. In agreement with this in vivo activity, curcumin suppressed compound 48/80-induced rat peritoneal mast cell (RPMC) degranulation and histamine release from RPMCs. Moreover, compound 48/80-elicited calcium uptake into RPMCs was reduced in a dose-dependent manner by curcumin. Furthermore, curcumin increased the level of intracellular cAMP and significantly inhibited the compound 48/80-induced reduction of cAMP in RPMCs. These results corroborate the finding that curcumin may have anti-allergic activity.
Keyword
MeSH Terms
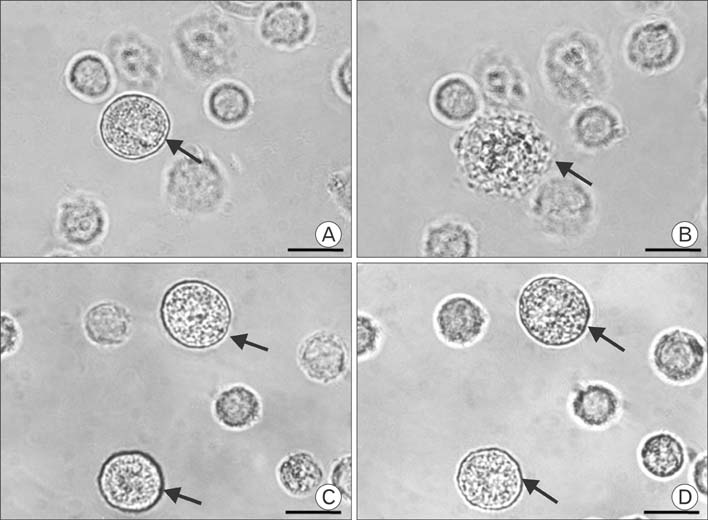
Figure
Cited by 1 articles
-
Antipruritic effect of curcumin on histamine-induced itching in mice
Han Kyu Lee, Seok Bum Park, Su-youne Chang, Sung Jun Jung
Korean J Physiol Pharmacol. 2018;22(5):547-554. doi: 10.4196/kjpp.2018.22.5.547.
Reference
-
1. Akagi M, Katakuse Y, Fukuishi N, Kan T, Akagi R. Superoxide anion-induced histamine release from rat peritoneal mast cells. Biol Pharm Bull. 1994. 17:732–734.2. Allansmith MR, Baird RS, Ross RN, Barney NP, Bloch KJ. Ocular anaphylaxis induced in the rat by topical application of compound 48/80. Dose response and time course study. Acta Ophthalmol Suppl. 1989. 192:145–153.3. Choi YH, Yan GH, Chai OH, et al. Inhibition of anaphylaxis-like reaction and mast cell activation by water extract from the fruiting body of Phellinus linteus. Biol Pharm Bull. 2006a. 29:1360–1365.4. Choi YH, Yan GH, Chai OH, et al. Inhibitory effects of Agaricus blazei on mast cell-mediated anaphylaxis-like reactions. Biol Pharm Bull. 2006b. 29:1366–1371.5. Cochrane DE, Douglas WW. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci USA. 1974. 71:408–412.6. Cui SX, Qu XJ, Xie YY, et al. Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med. 2006. 18:227–231.7. Ennis M, Pearce FL, Weston PM. Some studies on the release of histamine from mast cells stimulated with polylysine. Br J Pharmacol. 1980. 70:329–334.8. Eybl V, Kotyzova D, Koutensky J. Comparative study of natural antioxidants - curcumin, resveratrol and melatonin - in cadmium-induced oxidative damage in mice. Toxicology. 2006. 225:150–156.9. Fukuishi N, Sakaguchi M, Matsuura S, Nakagawa C, Akagi R, Akagi M. The mechanisms of compound 48/80-induced superoxide generation mediated by A-kinase in rat peritoneal mast cells. Biochem Mol Med. 1997. 61:107–113.10. Galli SJ. New concepts about the mast cell. N Engl J Med. 1993. 328:257–265.11. Hachisuka H, Nomura H, Sakamoto F, Mori O, Okubo K, Sasai Y. Effect of antianaphylactic agents on substance-P induced histamine release from rat peritoneal mast cells. Arch Dermatol Res. 1988. 280:158–162.12. Haddad JJ, Safieh-Garabedian B, Saade NE, et al. Chemioxyexcitation (delta pO2/ROS)-dependent release of IL-1 beta, IL-6 and TNF-alpha: evidence of cytokines as oxygen-sensitive mediators in the alveolar epithelium. Cytokine. 2001. 13:138–147.13. Harvima RJ, Harvima IT, Fräki JE. Optimization of histamine radio enzyme assay with purified histamine-N-methyltransferase. Clin Chim Acta. 1988. 171:247–256.14. Holmegaard SN. Measurement of cyclic AMP in clinical investigations. Acta Endocrinol Suppl (Copenh). 1982. 249:1–47.15. Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993. 465:359–386.16. Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994. 1224:255–263.17. Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008. 9:1215–1223.18. Kaliner M, Austen KF. Cyclic AMP, ATP, and reversed anaphylactic histamine release from rat mast cells. J Immunol. 1974. 112:664–674.19. Kurup VP, Barrios CS. Immunomodulatory effects of curcumin in allergy. Mol Nutr Food Res. 2008. 52:1031–1039.20. Lee JH, Kim JW, Ko NY, et al. Curcumin, a constituent of curry, suppresses IgE-mediated allergic response and mast cell activation at the level of Syk. J Allergy Clin Immunol. 2008. 121:1225–1231.21. Matsuda H, Tewtrakul S, Morikawa T, Nakamura A, Yoshikawa M. Anti-allergic principles from Thai zedoary: structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-23 cells. Bioorg Med Chem. 2004. 12:5891–5898.22. Nugroho AE, Ikawati Z, Sardjiman , Maeyama K. Effects of benzylidencyclopentanone analogues of curcumine on histamine release from mast cells. Biol Pharm Bull. 2009. 32:842–849.23. Palomäki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol. 2006. 147:596–606.24. Petersen LJ, Mosbech H, Skov PS. Allergen-induced histamine release in intact human skin in vivo assessed by skin microdialysis technique: characterization of factors influencing histamine releasability. J Allergy Clin Immunol. 1996. 97:672–679.25. Suzuki M, Nakamura T, Iyoki S, et al. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005. 28:1438–1443.26. Tasaka K, Mio M, Okamoto M. Intracellular calcium release induced by histamine releasers and its inhibition by some antiallergic drugs. Ann Allergy. 1986. 56:464–469.27. Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999. 402s:6760 Suppl. B24–B30.28. Weston MC, Peachell PT. Regulation of human mast cell and basophil function by cAMP. Gen Pharmacol. 1998. 31:715–719.29. Yoshii N, Mio M, Tasaka K. Ca uptake and Ca releasing properties of the endoplasmic reticulum in rat peritoneal mast cells. Immunopharmacology. 1988. 16:107–113.30. Yoshimura T, Hamaguchi E, Usami E, et al. Increased in vitro release of interferon-gamma from ampicillin-stimulated peripheral blood mononuclear cells in Stevens-Johnson syndrome. Biol Pharm Bull. 2004. 27:929–931.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of epigallocatechin gallate on compound 48/80-inducedmast cell activation and passive cutaneous anaphylaxis
- Inhibitory Effect of Corni fructus on Compound 48/80-induced Mast Cell Activation and Vascular Permeability
- Inhibitory Effect of Arctium lappa Linne on Compound 48/80-induced Mast Cell Activation and Vascular Permeability
- Inhibitory Effect of Rubus Coreanus on Compound 48/80- or Anti-DNP IgE-Induced Mast Cell Activation
- Inhibitory Effect of Polysaccharide Fraction from Cortex Mori on Compound 48/80-Induced Mast Cell Activation