Anat Cell Biol.
2010 Jun;43(2):97-109. 10.5115/acb.2010.43.2.97.
Re-engineering the mitochondrial genomes in mammalian cells
- Affiliations
-
- 1Mitochondria Hub Regulation Center and Department of Anatomy and Cell Biology, Dong-A University, Busan, Korea. ygyoon@donga.ac.kr
- 2Institute of Human Genetics and Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, USA.
- KMID: 2168882
- DOI: http://doi.org/10.5115/acb.2010.43.2.97
Abstract
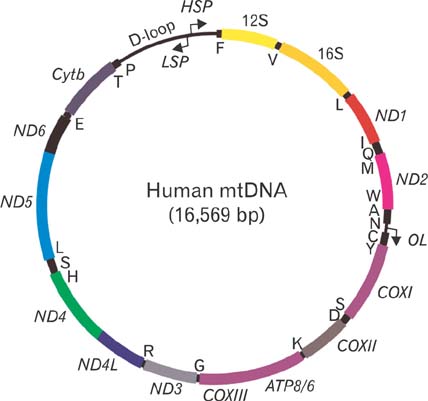
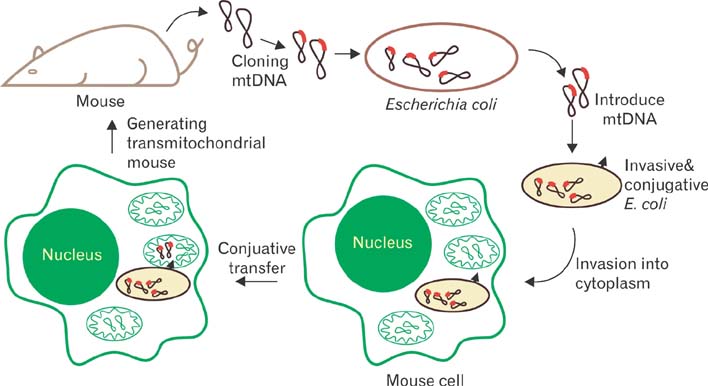
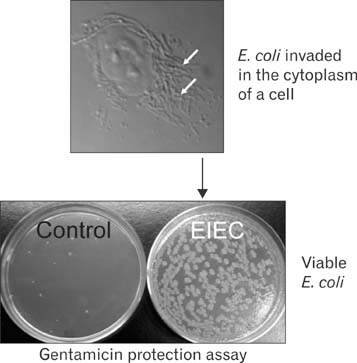
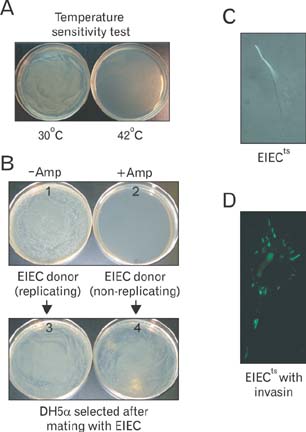
- Mitochondria are subcellular organelles composed of two discrete membranes in the cytoplasm of eukaryotic cells. They have long been recognized as the generators of energy for the cell and also have been known to associate with several metabolic pathways that are crucial for cellular function. Mitochondria have their own genome, mitochondrial DNA (mtDNA), that is completely separated and independent from the much larger nuclear genome, and even have their own system for making proteins from the genes in this mtDNA genome. The human mtDNA is a small (~16.5 kb) circular DNA and defects in this genome can cause a wide range of inherited human diseases. Despite of the significant advances in discovering the mtDNA defects, however, there are currently no effective therapies for these clinically devastating diseases due to the lack of technology for introducing specific modifications into the mitochondrial genomes and for generating accurate mtDNA disease models. The ability to engineer the mitochondrial genomes would provide a powerful tool to create mutants with which many crucial experiments can be performed in the basic mammalian mitochondrial genetic studies as well as in the treatment of human mtDNA diseases. In this review we summarize the current approaches associated with the correction of mtDNA mutations in cells and describe our own efforts for introducing engineered mtDNA constructs into the mitochondria of living cells through bacterial conjugation.
Keyword
MeSH Terms
Figure
Reference
-
1. Alam TI, Kanki T, Muta T, et al. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 2003. 31:1640–1645.2. Alexeyev MF, Venediktova N, Pastukh V, Shokolenko I, Bonilla G, Wilson GL. Selective elimination of mutant mitochondrial genomes as therapeutic strategy for the treatment of NARP and MILS syndromes. Gene Ther. 2008. 15:516–523.3. Becker S, Theile S, Heppeler N, et al. A generic system for the Escherichia coli cell-surface display of lipolytic enzymes. FEBS Lett. 2005. 579:1177–1182.4. Boddapati SV, Tongcharoensirikul P, Hanson RN, D'Souza GG, Torchilin VP, Weissig V. Mitochondriotropic liposomes. J Liposome Res. 2005. 15:49–58.5. Castellani R, Hirai K, Aliev G, et al. Role of mitochondrial dysfunction in Alzheimer's disease. J Neurosci Res. 2002. 70:357–360.6. Chen LB, Summerhayes IC, Johnson LV, Walsh ML, Bernal SD, Lampidis TJ. Probing mitochondria in living cells with rhodamine 123. Cold Spring Harb Symp Quant Biol. 1982. 46(Pt 1):141–155.7. Clark KM, Brown TA, Davidson MM, Papadopoulou LC, Clayton DA. Differences in nuclear gene expression between cells containing monomer and dimer mitochondrial genomes. Gene. 2002. 286:91–104.8. Collombet JM, Wheeler VC, Vogel F, Coutelle C. Introduction of plasmid DNA into isolated mitochondria by electroporation. A novel approach toward gene correction for mitochondrial disorders. J Biol Chem. 1997. 272:5342–5347.9. DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. Am J Med Genet. 2001. 106:18–26.10. DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003. 348:2656–2668.11. D'Souza GG, Boddapati SV, Weissig V. Mitochondrial leader sequence--plasmid DNA conjugates delivered into mammalian cells by DQAsomes co-localize with mitochondria. Mitochondrion. 2005. 5:352–358.12. D'Souza GG, Rammohan R, Cheng SM, Torchilin VP, Weissig V. DQAsome-mediated delivery of plasmid DNA toward mitochondria in living cells. J Control Release. 2003. 92:189–197.13. Edgar D, Shabalina I, Camara Y, et al. Random point mutations with major effects on protein-coding genes are the driving force behind premature aging in mtDNA mutator mice. Cell Metab. 2009. 10:131–138.14. Epler JL, Shugart LR, Barnett WE. N-formylmethionyl transfer ribonucleic acid in mitochondria from Neurospora. Biochemistry. 1970. 9:3575–3579.15. Flierl A, Jackson C, Cottrell B, Murdock D, Seibel P, Wallace DC. Targeted delivery of DNA to the mitochondrial compartment via import sequence-conjugated peptide nucleic acid. Mol Ther. 2003. 7:550–557.16. Fox TD, Sanford JC, McMullin TW. Plasmids can stably transform yeast mitochondria lacking endogenous mtDNA. Proc Natl Acad Sci U S A. 1988. 85:7288–7292.17. Frey TG, Mannella CA. The internal structure of mitochondria. Trends Biochem Sci. 2000. 25:319–324.18. Galper JB, Darnell JE. The presence of N-formyl-methionyl-tRNA in HeLa cell mitochondria. Biochem Biophys Res Commun. 1969. 34:205–214.19. Geromel V, Cao A, Briane D, et al. Mitochondria transfection by oligonucleotides containing a signal peptide and vectorized by cationic liposomes. Antisense Nucleic Acid Drug Dev. 2001. 11:175–180.20. Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999. 283:1476–1481.21. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998. 281:1309–1312.22. Grillot-Courvalin C, Goussard S, Huetz F, Ojcius DM, Courvalin P. Functional gene transfer from intracellular bacteria to mammalian cells. Nat Biotechnol. 1998. 16:862–866.23. Hashimoto K, Angiolillo P, Rottenberg H. Membrane potential and surface potential in mitochondria. Binding of a cationic spin probe. Biochim Biophys Acta. 1984. 764:55–62.24. Heinemann JA, Sprague GF Jr. Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989. 340:205–209.25. Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988. 331:717–719.26. Johnston SA, Anziano PQ, Shark K, Sanford JC, Butow RA. Mitochondrial transformation in yeast by bombardment with microprojectiles. Science. 1988. 240:1538–1541.27. Keeney PM, Quigley CK, Dunham LD, et al. Mitochondrial gene therapy augments mitochondrial physiology in a Parkinson's disease cell model. Hum Gene Ther. 2009. 20:897–907.28. Khan SM, Bennett JP Jr. Development of mitochondrial gene replacement therapy. J Bioenerg Biomembr. 2004. 36:387–393.29. Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A. 1996. 93:1156–1160.30. King MP, Attardi G. Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell. 1988. 52:811–819.31. King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989. 246:500–503.32. Koulintchenko M, Temperley RJ, Mason PA, Dietrich A, Lightowlers RN. Natural competence of mammalian mitochondria allows the molecular investigation of mitochondrial gene expression. Hum Mol Genet. 2006. 15:143–154.33. Kunik T, Tzfira T, Kapulnik Y, Gafni Y, Dingwall C, Citovsky V. Genetic transformation of HeLa cells by Agrobacterium. Proc Natl Acad Sci U S A. 2001. 98:1871–1876.34. Lee M, Choi JS, Choi MJ, Pak YK, Rhee BD, Ko KS. DNA delivery to the mitochondria sites using mitochondrial leader peptide conjugated polyethylenimine. J Drug Target. 2007. 15:115–122.35. Legros F, Lombés A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002. 13:4343–4354.36. Lim YM, de Groof AJ, Bhattacharjee MK, Figurski DH, Schon EA. Bacterial conjugation in the cytoplasm of mouse cells. Infect Immun. 2008. 76:5110–5119.37. Lu Y, Beavis AD. Effect of leader peptides on the permeability of mitochondria. J Biol Chem. 1997. 272:13555–13561.38. Minczuk M, Papworth MA, Miller JC, Murphy MP, Klug A. Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA. Nucleic Acids Res. 2008. 36:3926–3938.39. Mukhopadhyay A, Heard TS, Wen X, Hammen PK, Weiner H. Location of the actual signal in the negatively charged leader sequence involved in the import into the mitochondrial matrix space. J Biol Chem. 2003. 278:13712–13718.40. Muratovska A, Lightowlers RN, Taylor RW, et al. Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: implications for mitochondrial DNA replication, expression and disease. Nucleic Acids Res. 2001. 29:1852–1863.41. Nass MM. Abnormal DNA patterns in animal mitochondria: ethidium bromide-induced breakdown of closed circular DNA and conditions leading to oligomer accumulation. Proc Natl Acad Sci U S A. 1970. 67:1926–1933.42. Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991. 254:1497–1500.43. Nishikawa M, Yoshida K. Trans-kingdom conjugation offers a powerful gene targeting tool in yeast. Genet Anal. 1998. 14:65–73.44. Panov AV, Gutekunst CA, Leavitt BR, et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002. 5:731–736.45. Parisi MA, Clayton DA. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991. 252:965–969.46. Perkins GA, Sun MG, Frey TG. Correlated light and electron microscopy/electron tomography of mitochondria in situ. Methods Enzymol. 2009. 456:29–52.47. Piers KL, Heath JD, Liang X, Stephens KM, Nester EW. Agrobacterium tumefaciens-mediated transformation of yeast. Proc Natl Acad Sci U S A. 1996. 93:1613–1618.48. Ross MF, Da Ros T, Blaikie FH, et al. Accumulation of lipophilic dications by mitochondria and cells. Biochem J. 2006. 400:199–208.49. Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984. 81:127–138.50. Saraste M. Oxidative phosphorylation at the fin de siecle. Science. 1999. 283:1488–1493.51. Scheffler IE. Mitochondria make a come back. Adv Drug Deliv Rev. 2001. 49:3–26.52. Seibel P, Trappe J, Villani G, Klopstock T, Papa S, Reichmann H. Transfection of mitochondria: strategy towards a gene therapy of mitochondrial DNA diseases. Nucleic Acids Res. 1995. 23:10–17.53. Shoubridge EA. A debut for mito-mouse. Nat Genet. 2000. 26:132–134.54. Sinai AP, Bavoil PM. Hyper-invasive mutants define a novel Pho-regulated invasion pathway in Escherichia coli. Mol Microbiol. 1993. 10:1125–1137.55. Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003. 100:5407–5412.56. Srivastava S, Moraes CT. Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum Mol Genet. 2001. 10:3093–3099.57. Sutton MD, Kaguni JM. Novel alleles of the Escherichia coli dnaA gene. J Mol Biol. 1997. 271:693–703.58. Tanaka M, Borgeld HJ, Zhang J, et al. Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J Biomed Sci. 2002. 9:534–541.59. Thorburn DR, Dahl HH. Mitochondrial disorders: genetics, counseling, prenatal diagnosis and reproductive options. Am J Med Genet. 2001. 106:102–114.60. Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004. 429:417–423.61. Vestweber D, Schatz G. DNA-protein conjugates can enter mitochondria via the protein import pathway. Nature. 1989. 338:170–172.62. von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986. 5:1335–1342.63. Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999. 283:1482–1488.64. Wallace DC. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu Rev Biochem. 2007. 76:781–821.65. Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988. 242:1427–1430.66. Waters VL. Conjugative transfer in the dissemination of β-lactam and aminoglycoside resistance. Front Biosci. 1999. 4:D433–D456.67. Waters VL. Conjugation between bacterial and mammalian cells. Nat Genet. 2001. 29:375–376.68. Waxman DJ, Strominger JL. Penicillin-binding proteins and the mechanism of action of β-lactam antibiotics. Annu Rev Biochem. 1983. 52:825–869.69. Weissig V, Cheng SM, D'Souza GG. Mitochondrial pharmaceutics. Mitochondrion. 2004. 3:229–244.70. Weissig V, Lasch J, Erdos G, Meyer HW, Rowe TC, Hughes J. DQAsomes: a novel potential drug and gene delivery system made from Dequalinium. Pharm Res. 1998. 15:334–337.71. Weissig V, Torchilin VP. Mitochondriotropic cationic vesicles: a strategy towards mitochondrial gene therapy. Curr Pharm Biotechnol. 2000. 1:325–346.72. Wu J, Kandavelou K, Chandrasegaran S. Custom-designed zinc finger nucleases: what is next? Cell Mol Life Sci. 2007. 64:2933–2944.73. Yoon YG, Koob MD. Efficient cloning and engineering of entire mitochondrial genomes in Escherichia coli and transfer into transcriptionally active mitochondria. Nucleic Acids Res. 2003. 31:1407–1415.74. Yoon YG, Koob MD. Transformation of isolated mammalian mitochondria by bacterial conjugation. Nucleic Acids Res. 2005. 33:e139.75. Yoon YG, Koob MD. Selection by drug resistance proteins located in the mitochondria of mammalian cells. Mitochondrion. 2008. 8:345–351.76. Yoon YG, Yang YW, Koob MD. PCR-based cloning of the complete mouse mitochondrial genome and stable engineering in Escherichia coli. Biotechnol Lett. 2009. 31:1671–1676.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Back to the Ends: Chromosomal DNA
- MitGEN: Single Nucleotide Polymorphism DB Browser for Human Mitochondrial Genome
- Mechanisms of Uniparental Mitochondrial DNA Inheritance in Cryptococcus neoformans
- Complete Sequence of the Mitochondrial Genome of Spirometra ranarum: Comparison with S. erinaceieuropaei and S. decipiens
- Mitochondrial Genome Sequences of Spirometra erinaceieuropaei and S. decipiens (Cestoidea: Diphyllobothriidae)