Anat Cell Biol.
2011 Mar;44(1):25-34. 10.5115/acb.2011.44.1.25.
Immunochemical changes of calbindin, calretinin and SMI32 in ischemic retinas induced by increase of intraocular pressure and by middle cerebral artery occlusion
- Affiliations
-
- 1Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul, Korea. sujaoh@catholic.ac.kr
- KMID: 2168865
- DOI: http://doi.org/10.5115/acb.2011.44.1.25
Abstract
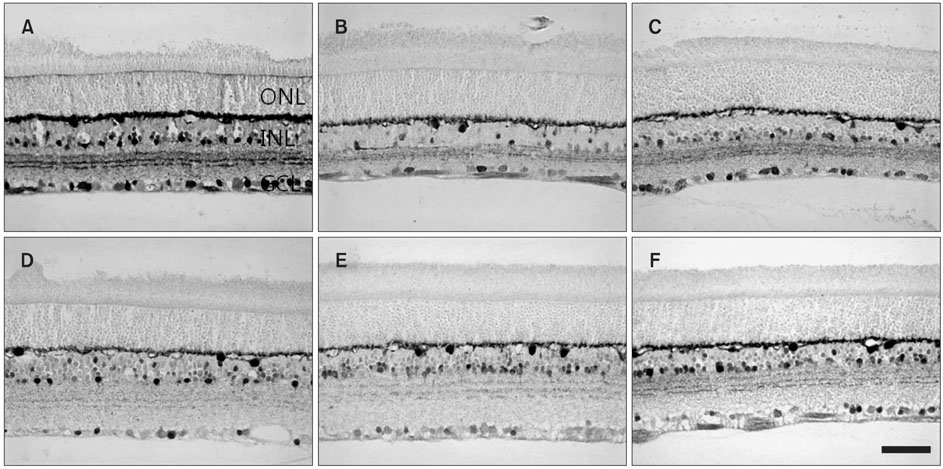
- The reaction of neuroactive substances to ischemic conditions in the rat retina evoked by different methods was immunochemically evaluated in adult Sprague-Dawley rats. Ocular ischemic conditions were unilaterally produced by elevating intraocular pressure (EIOP) or by middle cerebral artery occlusion (MCAO). Two EF-hand calcium binding proteins, calbindin D28K (CB) and calretinin (CR), in the normal retina showed similar immunolocalization, such as the amacrine and displaced amacrine cells, the ganglion cells, and their processes, particularly CB in horizontal cells. CB immunoreactive neurons in the ganglion cell layer in both types of ischemic retinas were more reduced in number than CR neurons compared to those in a normal retina. The CB protein level in both ischemic retinas was reduced to 60-80% of normal. The CR protein level in MCAO retinas was reduced to about 80% of normal but increased gradually to the normal value, whereas that in the EIOP showed a gradual reduction and a slight recovery. SMI32 immunoreactivity, which detects a dephosphorylated epitope of neurofilaments-M and -H, appeared in the axon bundles of ganglion cells in the innermost nerve fiber layer of normal retinas. The reactivity in the nerve fiber bundles appeared to only increase slightly in EIOP retinas, whereas a moderate increase occurred in MCAO retinas. The SMI32 protein level in MCAO retinas showed a gradual increasing tendency, whereas that in the EIOP showed a slight fluctuation. Interestingly, the MCAO retinas showed additional SMI32 immunoreactivity in the cell soma of presumed ganglion cells, whereas that of EIOP appeared in the Muller proximal radial fibers. Glial fibrillary acidic protein (GFAP) immunoreactivity appeared in the astrocytes located in the nerve fiber layer of normal retinas. Additional GFAP immunoreactivity appeared in the Muller glial fibers deep in EIOP retinas and at the proximal end in MCAO retinas. These findings suggest that the neurons in the ganglion cell layer undergo degenerative changes in response to ischemia, although EIOP retinas represented a remarkable Muller glial reaction, whereas MCAO retinas had only a small-scaled axonal transport disturbance.
Keyword
MeSH Terms
-
Adult
Amacrine Cells
Animals
Astrocytes
Axonal Transport
Axons
Calcium-Binding Protein, Vitamin D-Dependent
Calcium-Binding Proteins
Carisoprodol
Ganglion Cysts
Glial Fibrillary Acidic Protein
Humans
Infarction, Middle Cerebral Artery
Intraocular Pressure
Ischemia
Middle Cerebral Artery
Nerve Fibers
Neurons
Rats
Rats, Sprague-Dawley
Reference Values
Retina
Calcium-Binding Protein, Vitamin D-Dependent
Calcium-Binding Proteins
Carisoprodol
Glial Fibrillary Acidic Protein
Figure
Reference
-
1. Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol. 1989. 107:186–188.2. Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000. 41:431–442.3. Johnson EC, Morrison JC. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma. 2009. 18:341–353.4. Kim IB, Kim KY, Joo CK, Lee MY, Oh SJ, Chung JW, Chun MH. Reaction of Muller cells after increased intraocular pressure in the rat retina. Exp Brain Res. 1998. 121:419–424.5. Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003. 230:263–290.6. Büchi ER. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992. 55:605–613.7. Ahmed FA, Chaudhary P, Sharma SC. Effects of increased intraocular pressure on rat retinal ganglion cells. Int J Dev Neurosci. 2001. 19:209–218.8. Dijk F, Kamphuis W. An immunocytochemical study on specific amacrine cell subpopulations in the rat retina after ischemia. Brain Res. 2004. 1026:205–217.9. Dijk F, van Leeuwen S, Kamphuis W. Differential effects of ischemia/reperfusion on amacrine cell subtype-specific transcript levels in the rat retina. Brain Res. 2004. 1026:194–204.10. Kwon OJ, Kim JY, Kim SY, Jeon CJ. Alterations in the localization of calbindin D28K-, calretinin-, and parvalbumin-immunoreactive neurons of rabbit retinal ganglion cell layer from ischemia and reperfusion. Mol Cells. 2005. 19:382–390.11. Du Y, Hirooka K, Miyamoto O, Itano T, Tokuda M, Shiraga F. Both amacrine and bipolar cells release glutamate in the rat retina after ischemia/reperfusion insult in vitro. Curr Eye Res. 2008. 33:782–788.12. Sucher NJ, Lipton SA, Dreyer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997. 37:3483–3493.13. Naskar R, Dreyer EB. New horizons in neuroprotection. Surv Ophthalmol. 2001. 45:Suppl 3. S250–S255.14. Ullian EM, Barkis WB, Chen S, Diamond JS, Barres BA. Invulnerability of retinal ganglion cells to NMDA excitotoxicity. Mol Cell Neurosci. 2004. 26:544–557.15. Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999. 43:Suppl 1. S102–S128.16. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004. 363:1711–1720.17. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989. 20:84–91.18. Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951. 193:265–275.19. Peterson GL. Review of the Folin phenol protein quantitation method of Lowry, Rosenbrough, Farr and Randall. Anal Biochem. 1979. 100:201–220.20. Rogers JH. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol. 1987. 105:1343–1353.21. Mojumder DK, Wensel TG, Frishman LJ. Subcellular compartmentalization of two calcium binding proteins, calretinin and calbindin-28 kDa, in ganglion and amacrine cells of the rat retina. Mol Vis. 2008. 14:1600–1613.22. Kim SA, Jeon JH, Son MJ, Cha J, Chun MH, Kim IB. Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina. Anat Cell Biol. 2010. 43:218–229.23. Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992. 15:303–308.24. Hwang IK, Kang TC, Lee JC, Park SK, An SJ, Lee IS, Lee YB, Sohn HS, Kang JH, Choi SY, Won MH. Chronological alterations of calbindin D-28k immunoreactivity in the gerbil main olfactory bulb after ischemic insult. Brain Res. 2003. 971:250–254.25. Lee JC, Hwang IK, Yoo KY, Jung JY, Cho JH, Moon SM, Kang TC, Kim WK, Kim YS, Won MH. Calbindin D-28k is expressed in the microvascular basal lamina in the ventral horn at early time after transient spinal cord ischemia in the rabbit. Brain Res. 2005. 1047:123–128.26. D'Orlando C, Fellay B, Schwaller B, Salicio V, Bloc A, Gotzos V, Celio MR. Calretinin and calbindin D-28k delay the onset of cell death after excitotoxic stimulation in transfected P19 cells. Brain Res. 2001. 909:145–158.27. Wang X, Ng YK, Tay SS. Factors contributing to neuronal degeneration in retinas of experimental glaucomatous rats. J Neurosci Res. 2005. 82:674–689.28. Fan Y, Shi L, Gu Y, Zhao Y, Xie J, Qiao J, Yang GY, Wang Y, Lu CZ. Pretreatment with PTD-calbindin D 28k alleviates rat brain injury induced by ischemia and reperfusion. J Cereb Blood Flow Metab. 2007. 27:719–728.29. Soifer D, Nicoletti V, Cabane K, Mack K, Poulos B. Expression of the neurofilament protein NF-H in L cells. J Neurosci Res. 1991. 30:63–71.30. Lim EJ, Kim IB, Oh SJ, Chun MH. Identification and characterization of SMI32-immunoreactive amacrine cells in the mouse retina. Neurosci Lett. 2007. 424:199–202.31. Lin H, Zhai J, Cañete-Soler R, Schlaepfer WW. 3' untranslated region in a light neurofilament (NF-L) mRNA triggers aggregation of NF-L and mutant superoxide dismutase 1 proteins in neuronal cells. J Neurosci. 2004. 24:2716–2726.32. Meller D, Eysel UT, Schmidt-Kastner R. Transient immunohistochemical labelling of rat retinal axons during Wallerian degeneration by a monoclonal antibody to neurofilaments. Brain Res. 1994. 648:162–166.33. Kashiwagi K, Ou B, Nakamura S, Tanaka Y, Suzuki M, Tsukahara S. Increase in dephosphorylation of the heavy neurofilament subunit in the monkey chronic glaucoma model. Invest Ophthalmol Vis Sci. 2003. 44:154–159.34. Kalesnykas G, Tuulos T, Uusitalo H, Jolkkonen J. Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience. 2008. 155:937–947.35. Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009. 28:423–451.36. Steele EC Jr, Guo Q, Namura S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke. 2008. 39:2099–2104.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells
- Changes in transcript and protein levels of calbindin D28k, calretinin and parvalbumin, and numbers of neuronal populations expressing these proteins in an ischemia model of rat retina
- Intracranial Cerebrovascular Revascularization(Extracranial-Intracranial Arterial Bypass, EIAB)
- Phenylephrine - induced Hypertension Decreases the Area of Ischemia Following Middle Cerebral Artery Occlusion in the Rat
- An Experimental Study for Effect of Controlled Hypotension on Acute Ischemic Brain Lesion