J Bacteriol Virol.
2014 Jun;44(2):177-187. 10.4167/jbv.2014.44.2.177.
Proteome Analysis of a Catalase-deficient Isogenic Mutant of Helicobacter pylori 26695
- Affiliations
-
- 1Department of Microbiology, School of Medicine, Gyeongsang National University, Gyeongnam, Korea. wklee@gnu.kr
- 2Department of Pediatrics, School of Medicine, Gyeongsang National University, Gyeongnam, Korea.
- 3Research Institute of Life Science, Gyeongsang National University, Gyeongnam, Korea.
- KMID: 2168693
- DOI: http://doi.org/10.4167/jbv.2014.44.2.177
Abstract
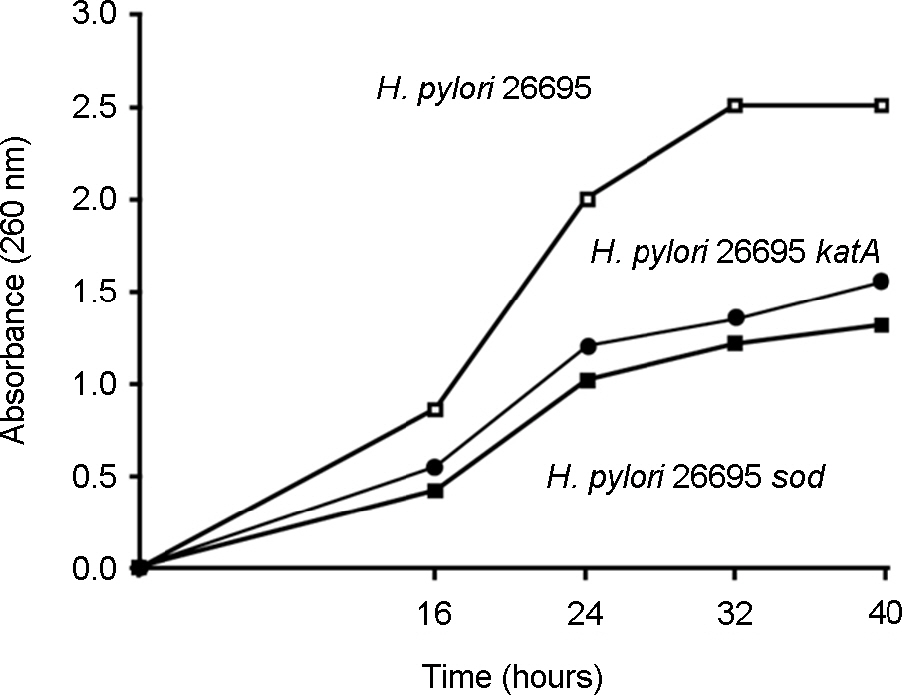
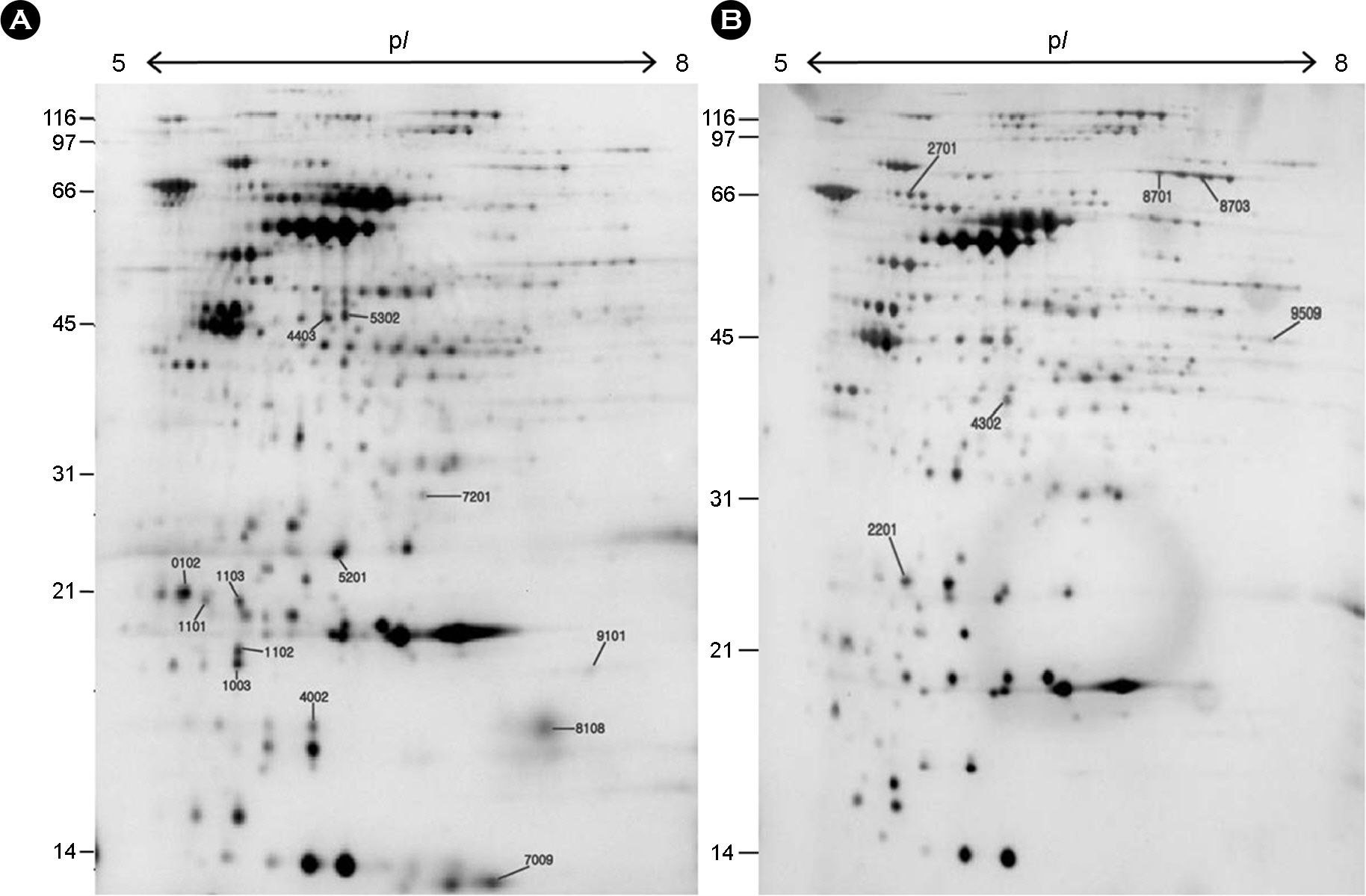
- Helicobacter pylori, a gram-negative bacterium, is a causative agent of gastroduodenal diseases of human. Human immune system produces harmful reactive oxygen species to kill this bacterium that locates the microaerophilic mucous layer. H. pylori harbors various antioxidant enzymes including SodB, KatA and AhpC to protect the oxygen toxicity. We removed the catalase gene (katA) from H. pylori 26695 genome, and the change of profile of the gene expression of the mutant was analyzed by high resolution 2-DE followed by matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), tandem MS and microarray analysis. Eleven and 37 genes were upregulated and downregulated in the mutant respectively, either transcriptionally or translationally. Expression level of pfr and hp1588 that were decreased on protein level in the mutant was confirmed by RT-PCR analysis.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Proteome Analysis of Alkylhydroxide Peroxidase-Deficient Isogenic Mutant of Helicobacter pylori 26695
Woo-Kon Lee, Seung-Chul Baik, Min-Kyung Shin, Myunghwan Jung, Jin-Sik Park, Jong-Hoon Ha, Dong-Hae Lee, Min-Jeong Kim, Jeong-ih Shin, Hyung-Lyun Kang
J Bacteriol Virol. 2019;49(4):191-202. doi: 10.4167/jbv.2019.49.4.191.
Reference
-
1). Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1:1311–5.
Article2). Nomura A, Stemmermann GN, Chyou PH, Perez-perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994; 120:977–81.3). Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991; 325:1132–6.4). No authors listed. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC monogr eval carcinog risks hum. 1994; 61:1–241.5). Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999; 284:1328–33.6). Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997; 388:539–47.7). Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999; 397:176–80.8). Brown PO, Botstein D. Exploring the new world of the genome with DNA microarrays. Nat Genet. 1999; 21:33–7.
Article9). Seyler RW Jr, Olson JW, Maier RJ. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect Immun. 2001; 69:4034–40.10). Ramarao N, Gray-Owen SD, Meyer TF. Helicobacter pylori induces but survives the extracellular release of oxygen radicals from professional phagocytes using its catalase activity. Mol Microbiol. 2000; 38:103–13.11). Basu M1, Czinn SJ, Blanchard TG. Absence of catalase reduces long-term survival of Helicobacter pylori in macrophage phagosomes. Helicobacter. 2004; 9:211–6.12). Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006; 61:847–60.13). Cho MJ, Lee SG, Lee KH, Song JY, Lee WK, Baik SC, et al. Comparison of gene expression patterns between Helicobacter pylor 26695 and its superoxide dismutase isogenic mutant. J Bacteriol Virol. 2013; 43:279–89.14). Alting-Mees MA, Short JM. pBluescript II: gene mapping vectors. Nucleic Acids Res. 1989; 17:9494.
Article15). Song JY, Park SG, Kang HL, Lee WK, Cho MJ, Park JU, et al. pHP489, a Helicobacter pylori small cryptic plasmid, harbors a novel gene coding for a replication initiation protein. Plasmid. 2003; 50:236–41.16). Wang Y, Roos KP, Taylor DE. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J Gen Microbiol. 1993; 139:2485–93.17). O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975; 250:4007–21.18). Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–54.
Article19). Heukeshoven J, Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988; 9:28–32.
Article20). Park JW, Song JY, Hwang HR, Park HJ, Youn HS, Seo JH, et al. Proteomic analysis of thiol-active proteins of Helicobacter pylori 26695. J Bacteriol Virol. 2012; 42:211–23.21). Chuang SE, Daniels DL, Blattner FR. Global regulation of gene expression in Escherichia coli. J Bacteriol. 1993; 175:2026–36.22). Tatusov RL, Koonin EV, Lipman DJ. A genomic perspective on protein families. Science. 1997; 278:631–7.
Article23). Harris AG, Hinds FE, Beckhouse AG, Kolesnikow T, Hazell SL. Resistance to hydrogen peroxide in Helicobacter pylori: role of catalase (KatA) and Fur, and functional analysis of a novel gene product designated ‘KatA-associated protein’, KapA (HP0874). Microbiology. 2002; 148:3813–25.24). Yamada H, Shimizu S, Shimada H, Tani Y, Takahashi S, Ohashi T. Production of D-phenylglycine-related amino acids by immobilized microbial cells. Biochimie. 1980; 62:395–9.
Article25). Baik SC, Kim KM, Song SM, Kim DS, Jun JS, Lee SG, et al. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Helicobacter pylori strain 26695. J Bacteriol. 2004; 186:949–55.26). Hochhauser SJ, Weiss B. Escherichia coli mutants deficient in deoxyuridine triphosphatase. J Bacteriol. 1978; 134:157–66.27). Vicente JB, Ehrenkaufer GM, Saraiva LM, Teixeira M, Singh U. Entamoeba histolytica modulates a complex repertoire of novel genes in response to oxidative and nitrosative stresses: implications for amebic pathogenesis. Cell Microbiol. 2009; 11:51–69.28). Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Clarendon Press;1989.29). Slade D, Radman M. Oxidative Stress Resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev. 2011; 75:133–91.30). Skouloubris S, Labigne A, De Reuse H. Identification and characterization of an aliphatic amidase in Helicobacter pylori. Mol Microbiol. 1997; 25:989–98.31). Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006; 52:601–23.
Article32). Bereswill S, Waidner U, Odenbreit S, Lichte F, Fassbinder F, Bode G, et al. Structural, functional and mutational analysis of the pfr gene encoding a ferritin from Helicobacter pylori. Microbiology. 1998; 144:2505–16.33). Nakamura A, Park A, Nagata K, Sato EF, Kashiba M, Tamura T, et al. Oxidative cellular damage associated with transformation of Helicobacter pylori from a bacillary to a coccoid form. Free Radic Biol Med. 2000; 28:1611–8.34). Wang G, Maier RJ. An NADPH quinone reductase of Helicobacter pylori plays an important role in oxidative stress resistance and host colonization. Infect Immun. 2004; 72:1391–6.35). Waidner B, Greiner S, Odenbreit S, Kavermann H, Velayudhan J, Stähler F, et al. Essential role of ferritin Pfr in Helicobacter pylori iron metabolism and gastric colonization. Infect Immun. 2002; 70:3923–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proteome Analysis of Alkylhydroxide Peroxidase-Deficient Isogenic Mutant of Helicobacter pylori 26695
- Proteomics Approach in Helicobacter pylori Researches
- Comparison of Gene Expression Patterns between Helicobacter pylor 26695 and its Superoxide Dismutase Isogenic Mutant
- Application of Hemin-Agarose Affinity Chromatography to Enrich Proteome Components of Helicobacter pylori Strain 26695
- Proteomic Analysis of Thiol-active Proteins of Helicobacter pylori 26695