J Bacteriol Virol.
2014 Jun;44(2):121-132. 10.4167/jbv.2014.44.2.121.
The in vivo and in vitro Roles of Epithelial Pattern Recognition Receptors in Pneumococcal Infections
- Affiliations
-
- 1Department of Microbiology, Ewha Womans University School of Medicine, Seoul, Korea. jlim19@ewha.ac.kr
- KMID: 2168687
- DOI: http://doi.org/10.4167/jbv.2014.44.2.121
Abstract
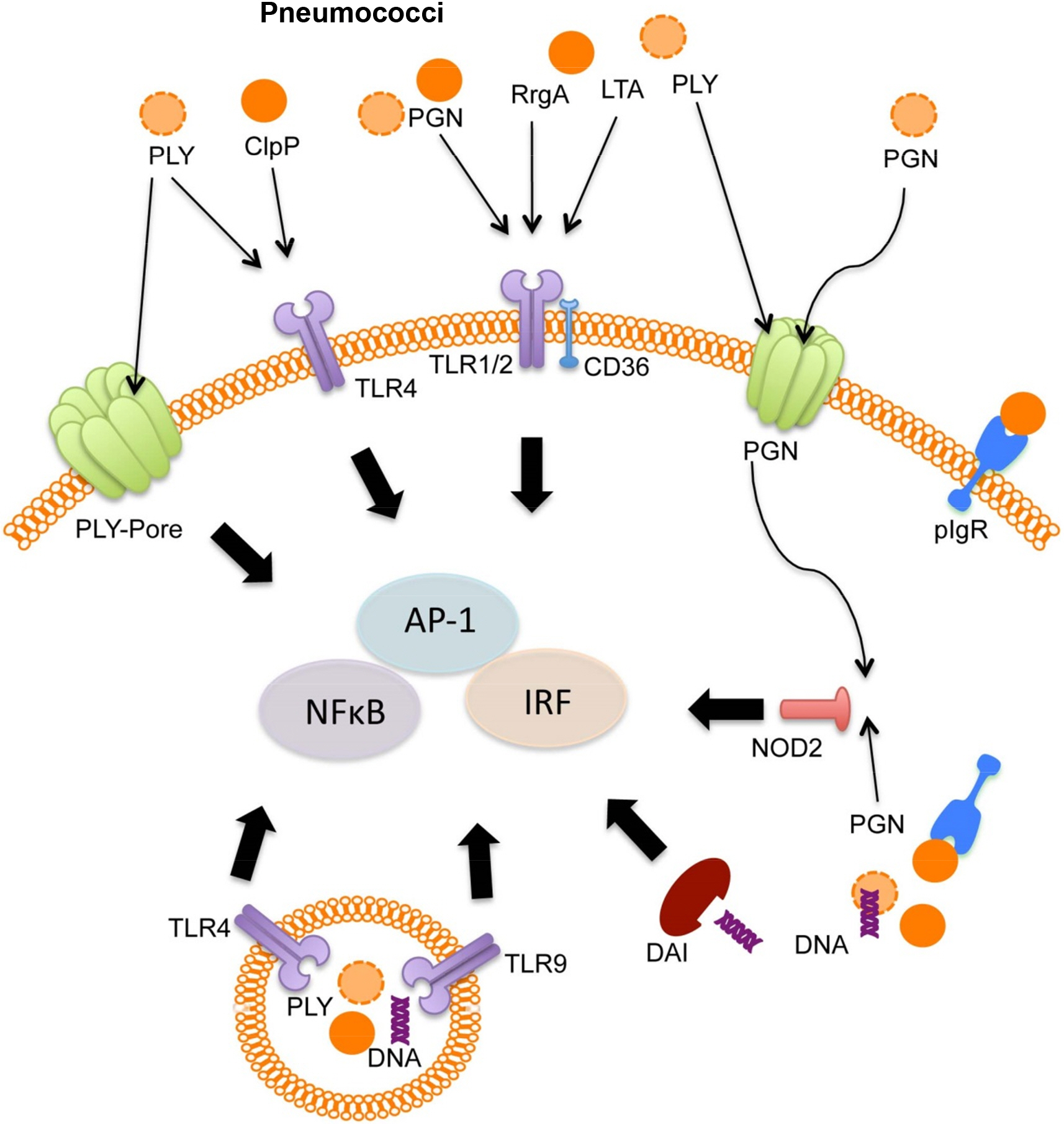
- Streptococcus pneumoniae, also called pneumococcus, is a major cause of infectious disease in human. Pneumococcus resides in the nasopharynx as an upper respiratory commensal, and most of pneumococcal colonizations are asymptomatic in immunocompetent individuals. When nasopharyngeal mucosal homeostasis is disrupted, pneumococcus migrates into middle ear and lower respiratory tract and causes detrimental colonization. In this regard, the epithelial cells of middle ear and lung act as first line of defense against pneumococcus to prevent invasive pneumococcal diseases. Respiratory epithelial cells express various cell-surface and intra-cellular receptors sensing microbial pathogens and respond to sensed pathogens by triggering intra-cellular signaling pathways and inducing pathogen-specific innate immune responses. Various epithelial cell-surface and intra-cellular receptors, such as Toll-like receptors (TLRs), Nod-like receptors (NLRs), intracellular DNA sensing receptors, and scavenger receptors (SRs), participate in sensing of pneumococcus, and the activation of these receptors by pneumococcal components induces anti-pneumococcal innate immune responses including epithelial apoptosis and inflammatory cytokine/chemokine expressions. Epithelial sensing of pneumococcus is a critical step for setting an early defense against pneumococcal infection, and also is required to recruit and activate innate immune cells and trigger adaptive immunity.
Keyword
MeSH Terms
-
Adaptive Immunity
Apoptosis
Colon
Communicable Diseases
DNA
Ear, Middle
Epithelial Cells
Homeostasis
Humans
Immunity, Innate
Inflammation
Lung
Nasopharynx
Pneumococcal Infections*
Receptors, Pattern Recognition*
Receptors, Scavenger
Respiratory System
Streptococcus pneumoniae
Toll-Like Receptors
DNA
Receptors, Pattern Recognition
Receptors, Scavenger
Toll-Like Receptors
Figure
Reference
-
1). Watson DA, Musher DM, Jacobson JW, Verhoef J. A brief history of the pneumococcus in biomedical research: a panoply of scientific discovery. Clin Infect Dis. 1993; 17:913–24.
Article2). Pneumococcal conjugate vaccine for childhood immunization–WHO position paper. Wkly Epidemiol Rec. 2007; 82:93–104.3). Kalin M. Pneumococcal serotypes and their clinical relevance. Thorax. 1998; 53:159–62.
Article4). van Dam JE, Fleer A, Snippe H. Immunogenicity and immunochemistry of Streptococcus pneumoniae capsular polysaccharides. Antonie Van Leeuwenhoek. 1990; 58:1–47.5). Sankilampi U, Herva E, Haikala R, Liimatainen O, Renkonen OV, Leinonen M. Epidemiology of invasive Streptococcus pneumoniae infections in adults in Finland. Epidemiol Infect. 1997; 118:7–15.6). Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991; 325:1453–60.
Article7). Butler JC, Breiman RF, Lipman HB, Hofmann J, Facklam RR. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978–1994: implications for development of a conjugate vaccine. J Infect Dis. 1995; 171:885–9.8). Nielsen SV, Henrichsen J. Incidence of invasive pneumococcal disease and distribution of capsular types of pneumococci in Denmark, 1989–94. Epidemiol Infect. 1996; 117:411–6.
Article9). Scott JA, Hall AJ, Dagan R, Dixon JM, Eykyn SJ, Fenoll A, et al. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis. 1996; 22:973–81.10). Vitharsson G, Jónsdóttir I, Jónsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994; 170:592–9.11). Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010; 14:e197–209.12). Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011; 378:1962–73.
Article13). Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, et al. Effectiveness of sevenvalent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006; 368:1495–502.
Article14). Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010; 125:866–75.
Article15). Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine. 2011; 29:7207–11.16). Choi SY, Tran TD, Briles DE, Rhee DK. Inactivated pep27 mutant as an effective mucosal vaccine against a secondary lethal pneumococcal challenge in mice. Clin Exp Vaccine Res. 2013; 2:58–65.17). Daniels CC, Kim KH, Burton RL, Mirza S, Walker M, King J, et al. Modified opsonization, phagocytosis, and killing assays to measure potentially protective antibodies against pneumococcal surface protein A. Clin Vaccine Immunol. 2013; 20:1549–58.
Article18). Nguyen CT, Kim SY, Kim MS, Lee SE, Rhee JH. Intranasal immunization with recombinant PspA fused with a flagellin enhances cross-protective immunity against Streptococcus pneumoniae infection in mice. Vaccine. 2011; 29:5731–9.19). Janoff EN, Rubins JB. Invasive pneumococcal disease in the immunocompromised host. Microb Drug Resist. 1997; 3:215–32.
Article20). Sousa D, Justo I, Domínguez A, Manzur A, Izquierdo C, Ruiz L, et al. Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin Microbiol Infect. 2013; 19:187–92.
Article21). Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Raga-Luria X, Gomez-Bertomeu F. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med. 2009; 103:309–16.
Article22). Kalin M. Bacteremic pneumococcal pneumonia: value of culture of nasopharyngeal specimens and examination of washed sputum specimens. Eur J Clin Microbiol. 1982; 1:394–6.
Article23). Krantz I, Alestig K, Trollfors B, Zackrisson G. The carrier state in pertussis. Scand J Infect Dis. 1986; 18:121–3.
Article24). Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001; 43:182–6.
Article25). Lipsky BA, Boyko EJ, Inui TS, Koepsell TD. Risk factors for acquiring pneumococcal infections. Arch Intern Med. 1986; 146:2179–85.
Article26). Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000; 342:681–9.27). Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004; 23:S87–97.
Article28). Chuquimia OD, Petursdottir DH, Rahman MJ, Hartl K, Singh M, Fernández C. The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems. PLoS One. 2012; 7:e32125.
Article29). Gentry M, Taormina J, Pyles RB, Yeager L, Kirtley M, Popov VL, et al. Role of primary human alveolar epithelial cells in host defense against Francisella tularensis infection. Infect Immun. 2007; 75:3969–78.30). Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010; 11:656–65.
Article31). Broz P, Monack DM. Newly described pattern recognition receptors team up against intracellular pathogens. Nat Rev Immunol. 2013; 13:551–65.
Article32). Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011; 34:665–79.
Article33). Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012; 33:449–58.
Article34). Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008; 6:288–301.35). Koppe U, Suttorp N, Opitz B. Recognition of Streptococcus pneumoniae by the innate immune system. Cell Microbiol. 2012; 14:460–6.36). Greene CM, Carroll TP, Smith SG, Taggart CC, Devaney J, Griffin S, et al. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J Immunol. 2005; 174:1638–46.
Article37). Greene CM, McElvaney NG. Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz). 2005; 53:418–27.38). Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, et al. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004; 30:777–83.
Article39). Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007; 44:3100–11.
Article40). Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol. 2004; 30:627–34.41). Hershberg RM. The epithelial cell cytoskeleton and intracellular trafficking. V. Polarized compartmentalization of antigen processing and Toll-like receptor signaling in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G833–9.42). Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004; 5:190–8.
Article43). Platz J, Beisswenger C, Dalpke A, Koczulla R, Pinkenburg O, Vogelmeier C, et al. Microbial DNA induces a host defense reaction of human respiratory epithelial cells. J Immunol. 2004; 173:1219–23.
Article44). Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, et al. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007; 9:633–44.
Article45). Basset A, Zhang F, Benes C, Sayeed S, Herd M, Thompson C, et al. Toll-like receptor (TLR) 2 mediates inflammatory responses to oligomerized RrgA pneumococcal pilus type 1 protein. J Biol Chem. 2013; 288:2665–75.
Article46). Cao J, Gong Y, Dong S, Zhang L, Lai X, Zhang X, et al. Pneumococcal ClpP modulates the maturation and activation of human dendritic cells: implications for pneumococcal infections. J Leukoc Biol. 2013; 93:737–49.
Article47). Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A. 2003; 100:1966–71.
Article48). Moore LJ, Pridmore AC, Dower SK, Read RC. The glycopeptide vancomycin does not enhance toll-like receptor 2 (TLR2) activation by Streptococcus pneumoniae. J Antimicrob Chemother. 2004; 54:76–8.49). Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. 2005; 175:3084–91.50). Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999; 163:1–5.51). Karlström A, Heston SM, Boyd KL, Tuomanen EI, McCullers JA. Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J Infect Dis. 2011; 204:1358–66.
Article52). Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, et al. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep. 2004; 5:1000–6.
Article53). Dessing MC, Florquin S, Paton JC, van der Poll T. Tolllike receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell Microbiol. 2008; 10:237–46.
Article54). Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004; 173:7131–4.
Article55). Lammers AJ, de Porto AP, de Boer OJ, Florquin S, van der Poll T. The role of TLR2 in the host response to pneumococcal pneumonia in absence of the spleen. BMC Infect Dis. 2012; 12:139.
Article56). Echchannaoui H, Bachmann P, Letiembre M, Espinosa M, Landmann R. Regulation of Streptococcus pneumoniae distribution by Toll-like receptor 2 in vivo. Immunobiology. 2005; 210:229–36.57). Echchannaoui H, Leib SL, Neumann U, Landmann RM. Adjuvant TACE inhibitor treatment improves the outcome of TLR2-/- mice with experimental pneumococcal meningitis. BMC Infect Dis. 2007; 7:25.
Article58). Klein M, Obermaier B, Angele B, Pfister HW, Wagner H, Koedel U, et al. Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR) 2 and TLR4. J Infect Dis. 2008; 198:1028–36.
Article59). Letiembre M, Echchannaoui H, Ferracin F, Rivest S, Landmann R. Toll-like receptor-2 deficiency is associated with enhanced brain TNF gene expression during pneumococcal meningitis. J Neuroimmunol. 2005; 168:21–33.
Article60). Han F, Yu H, Tian C, Li S, Jacobs MR, Benedict-Alderfer C, et al. Role for Toll-like receptor 2 in the immune response to Streptococcus pneumoniae infection in mouse otitis media. Infect Immun. 2009; 77:3100–8.61). Li SL, Zhang MY, Li BY, Zheng QY, Zhu HL. Toll-like receptor 2 and Toll-like receptor 4 participates in mediation of acute otitis media and mortality in pneumococcal infections in mice. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011; 46:1009–18.62). Garnacho-Montero J, García-Cabrera E, Jiménez-Álvarez R, Díaz-Martín A, Revuelto-Rey J, Aznar-Martín J, et al. Genetic variants of the MBL2 gene are associated with mortality in pneumococcal sepsis. Diagn Microbiol Infect Dis. 2012; 73:39–44.
Article63). Moens L, Verhaegen J, Pierik M, Vermeire S, De Boeck K, Peetermans WE, et al. Toll-like receptor 2 and Tolllike receptor 4 polymorphisms in invasive pneumococcal disease. Microbes Infect. 2007; 9:15–20.
Article64). Tellería-Orriols JJ, García-Salido A, Varillas D, Serrano-González A, Casado-Flores J. TLR2-TLR4/CD14 polymorphisms and predisposition to severe invasive infections by Neisseria meningitidis and Streptococcus pneumoniae. Med Intensiva. 2013.65). Vuononvirta J, Toivonen L, Gröndahl-Yli-Hannuksela K, Barkoff AM, Lindholm L, Mertsola J, et al. Nasopharyngeal bacterial colonization and gene polymorphisms of mannose-binding lectin and toll-like receptors 2 and 4 in infants. PLoS One. 2011; 6:e26198.
Article66). Yuan FF, Marks K, Wong M, Watson S, de Leon E, McIntyre PB, Sullivan JS. Clinical relevance of TLR2, TLR4, CD14 and FcgammaRIIA gene polymorphisms in Streptococcus pneumoniae infection. Immunol Cell Biol. 2008; 86:268–70.67). Berenson CS, Kruzel RL, Eberhardt E, Dolnick R, Minderman H, Wallace PK, et al. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax. 2014.
Article68). Boyd AR, Shivshankar P, Jiang S, Berton MT, Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012; 47:507–18.
Article69). Fallah MP, Chelvarajan RL, Garvy BA, Bondada S. Role of phosphoinositide 3-kinase-Akt signaling pathway in the age-related cytokine dysregulation in splenic macrophages stimulated via TLR-2 or TLR-4 receptors. Mech Ageing Dev. 2011; 132:274–86.
Article70). van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005; 73:7718–26.71). Beisswenger C, Coyne CB, Shchepetov M, Weiser JN. Role of p38 MAP kinase and transforming growth factor-beta signaling in transepithelial migration of invasive bacterial pathogens. J Biol Chem. 2007; 282:28700–8.72). Bermpohl D, Halle A, Freyer D, Dagand E, Braun JS, Bechmann I, et al. Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J Clin Invest. 2005; 115:1607–15.
Article73). Sharif O, Matt U, Saluzzo S, Lakovits K, Haslinger I, Furtner T, et al. The scavenger receptor CD36 down-modulates the early inflammatory response while enhancing bacterial phagocytosis during pneumococcal pneumonia. J Immunol. 2013; 190:5640–8.
Article74). Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, et al. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun. 2004; 72:788–94.75). Ha U, Lim JH, Jono H, Koga T, Srivastava A, Malley R, et al. A novel role for IkappaB kinase (IKK) alpha and IKKbeta in ERK-dependent up-regulation of MUC5AC mucin transcription by Streptococcus pneumoniae. J Immunol. 2007; 178:1736–47.76). Lim JH, Ha U, Sakai A, Woo CH, Kweon SM, Xu H, et al. Streptococcus pneumoniae synergizes with nontypeable Haemophilus influenzae to induce inflammation via upregulating TLR2. BMC Immunol. 2008; 9:40.77). Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo CH, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007; 27:349–60.78). Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L428–37.
Article79). Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukoc Biol. 2006; 80:267–77.80). Ripoll VM, Kadioglu A, Cox R, Hume DA, Denny P. Macrophages from BALB/c and CBA/Ca mice differ in their cellular responses to Streptococcus pneumoniae. J Leukoc Biol. 2010; 87:735–41.81). Zahlten J, Steinicke R, Bertrams W, Hocke AC, Scharf S, Schmeck B, et al. TLR9- and Src-dependent expression of Krueppel-like factor 4 controls interleukin-10 expression in pneumonia. Eur Respir J. 2013; 41:384–91.
Article82). Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest. 2011; 121:3666–76.83). Moreira LO, El Kasmi KC, Smith AM, Finkelstein D, Fillon S, Kim YG, et al. The TLR2-MyD88-NOD2-RIPK2 signalling axis regulates a balanced pro-inflammatory and IL-10-mediated anti-inflammatory cytokine response to gram-positive cell walls. Cell Microbiol. 2008; 10:2067–77.
Article84). Opitz B, Püschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004; 279:36426–32.85). Liu X, Chauhan VS, Young AB, Marriott I. NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia. 2010; 58:839–47.86). Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010; 16:228–31.
Article87). Sorrentino R, de Souza PM, Sriskandan S, Duffin C, Paul-Clark MJ, Mitchell JA. Pattern recognition receptors and interleukin-8 mediate effects of Gram-positive and Gram-negative bacteria on lung epithelial cell function. Br J Pharmacol. 2008; 154:864–71.
Article88). Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, et al. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. MBio. 2011; 2:e00016–11.
Article89). Dorrington MG, Roche AM, Chauvin SE, Tu Z, Mossman KL, Weiser JN, et al. MARCO is required for TLR2- and Nod2-mediated responses to Streptococcus pneumoniae and clearance of pneumococcal colonization in the murine nasopharynx. J Immunol. 2013; 190:250–8.90). Sano H, Kuronuma K, Kudo K, Mitsuzawa H, Sato M, Murakami S, et al. Regulation of inflammation and bacterial clearance by lung collectins. Respirology. 2006; 11(Suppl):S46–50.
Article91). Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013; 34:317–28.
Article92). Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, et al. CYLD negatively regulates transforming growth factor-beta-signalling via deubiquitinating Akt. Nat Commun. 2012; 3:771.
Article93). Rijneveld AW, Florquin S, Bresser P, Levi M, De Waard V, Lijnen R, et al. Plasminogen activator inhibitor type-1 deficiency does not influence the outcome of murine pneumococcal pneumonia. Blood. 2003; 102:934–9.
Article94). Scharf S, Zahlten J, Szymanski K, Hippenstiel S, Suttorp N, N'Guessan PD. Streptococcus pneumoniae induces human beta-defensin-2 and -3 in human lung epithelium. Exp Lung Res. 2012; 38:100–10.95). Lim JH, Kim HJ, Komatsu K, Ha U, Huang Y, Jono H, et al. Differential regulation of Streptococcus pneumoniae-induced human MUC5AC mucin expression through distinct MAPK pathways. Am J Transl Res. 2009; 1:300–11.96). Schmeck B, Huber S, Moog K, Zahlten J, Hocke AC, Opitz B, et al. Pneumococci induced TLR- and Rac1-dependent NF-kappaB-recruitment to the IL-8 promoter in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006; 290:L730–L7.97). Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, et al. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005; 73:6479–87.
Article98). Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998; 282:2085–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NLRC4 Inflammasome-Mediated Regulation of Eosinophilic Functions
- Recent Advances in 'Human Rhinovirus and Allergy' Research
- The Innate Immune Responses in Pathogenesis of Chronic Rhinosinusitis
- Autophagy in Innate Recognition of Pathogens and Adaptive Immunity
- Activation of Innate Immune System During Viral Infection: Role of Pattern-recognition Receptors (PRRs) in Viral Infection