J Bacteriol Virol.
2009 Dec;39(4):329-336. 10.4167/jbv.2009.39.4.329.
Identification and Diagnostic Utility of Serologic Reactive Antigens from Mycobacterium tuberculosis Sonic Extracts
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chungnam National University, Deajeon, Korea. hjukim@cnu.ac.kr
- 2Division of Pulmonary, Sleep and Critical Care Medicine, Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea.
- 3Department of Laboratory Medicine, School of Medicine, Pusan National University, Yangsan, Korea.
- KMID: 2168544
- DOI: http://doi.org/10.4167/jbv.2009.39.4.329
Abstract
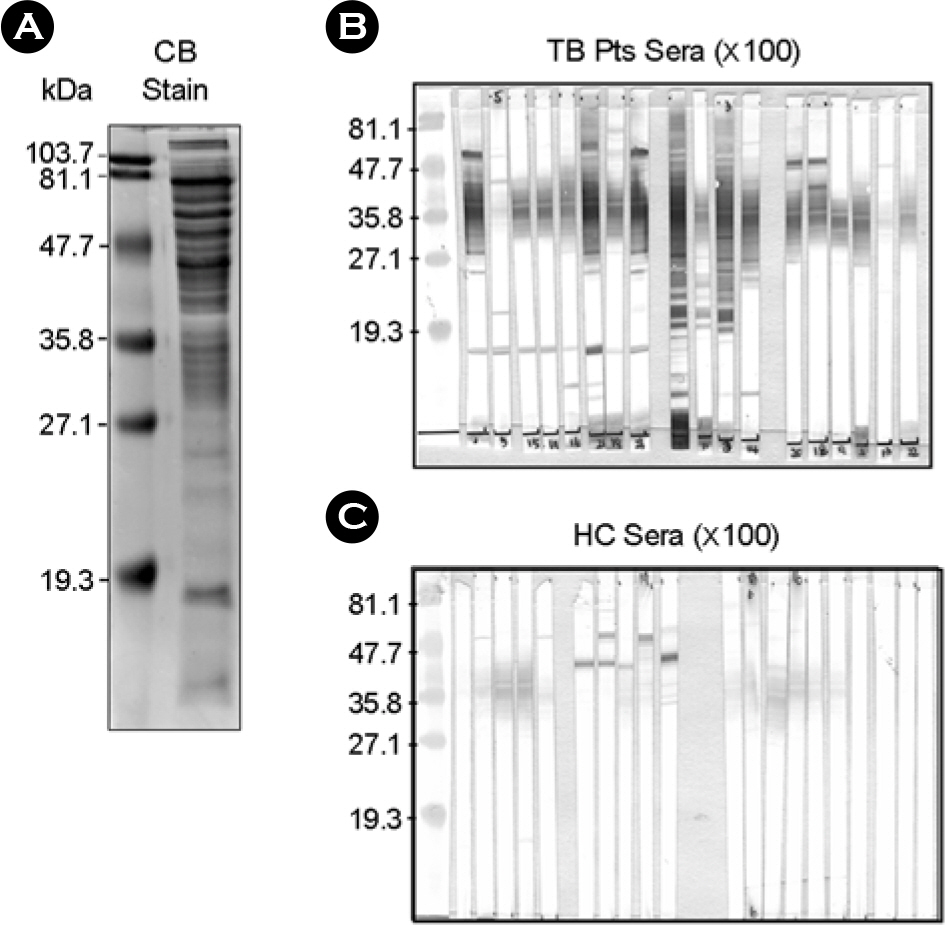
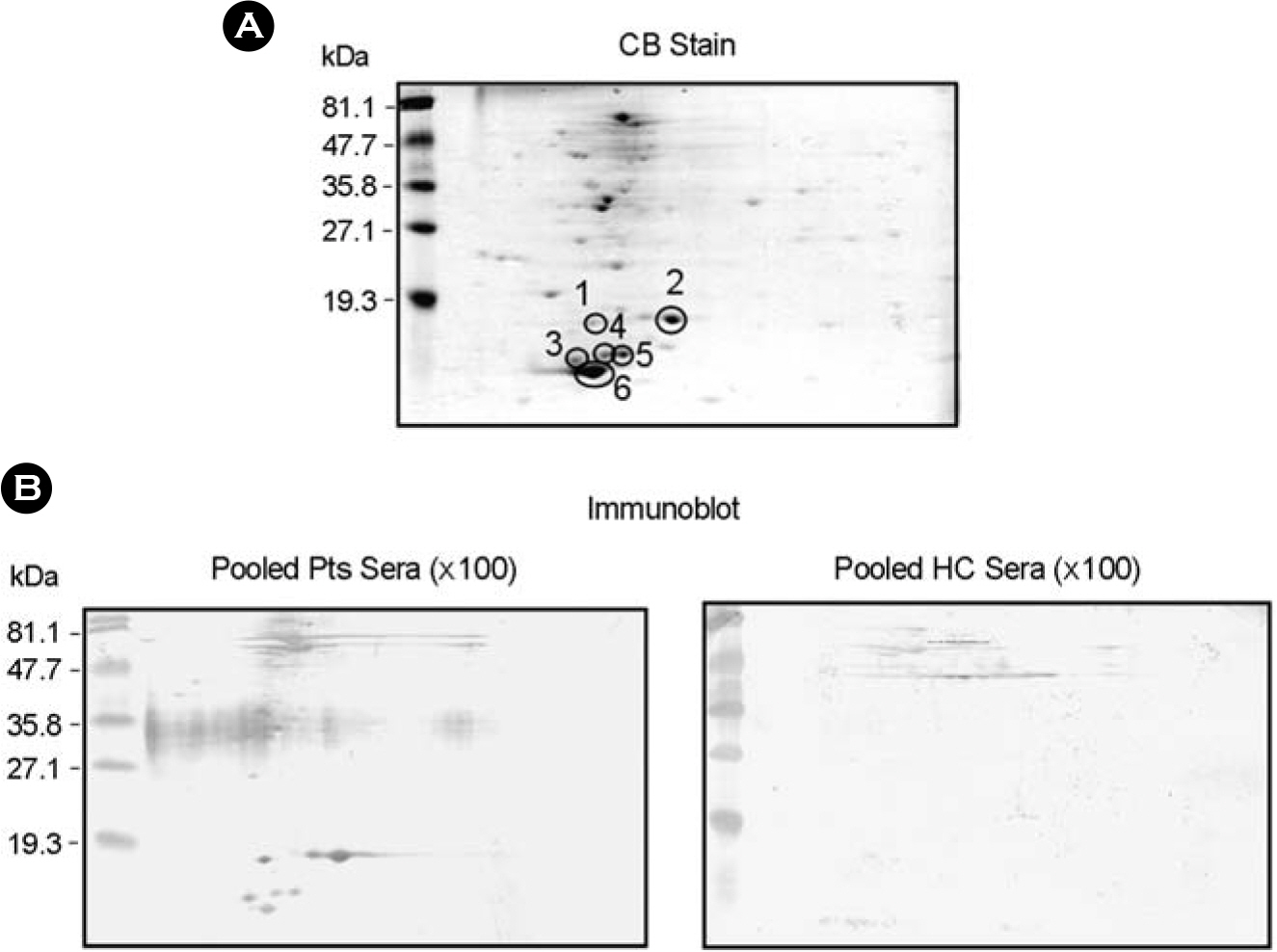
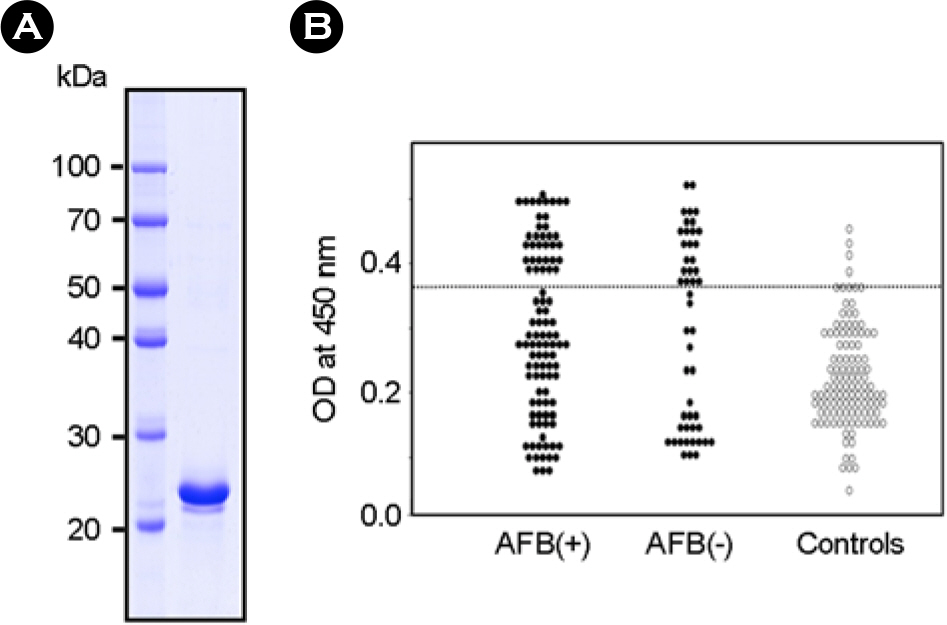
- It is important to identify and to test serologically active antigens, so as to devise a cocktail of the best antigens or peptides. We searched for antigens that have serodiagnostic utility using two-dimensional fractionation of sonic extracts from Mycobacterium tuberculosis and probing with pools of sera from healthy subjects and patients with tuberculosis (TB). Reactive protein spots with patient sera were identified by tandem mass spectrometry. Three proteins, Rv0652, Rv2626c, and Rv3418c, which have not previously been described as serologic targets, were identified. Rv0652 protein among them was expressed in Escherichia coli and serum IgG antibodies against this antigen were measured in 150 patients with pulmonary TB and in 115 healthy subjects. The sensitivity and specificity were 39% and 92%, respectively. These results suggest that a newly identified protein, Rv0652 may be a valuable candidate to be included in a cocktail test kit for TB diagnosis.
MeSH Terms
Figure
Reference
-
1). World Health Orgnaization. Global Tuberculosis Control-Surveilance, Planning, Financing, WHO report. 2005. Wolrd Health Organization;Geneva:2). Abebe F., Holm-Hansen C., Wiker HG., Bjune G. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand J Immunol. 2007. 66:176–91.3). Pai M., Kalantri S., Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: Part I. Latent tuberculosis. Expert Rev Mol Diagn. 2006. 6:413–22.
Article4). Lyashchenko K., Colangeli R., Houde M., Al Jahdali H., Menzies D., Gennaro ML. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998. 66:3936–40.
Article5). Steingart KR., Dendukuri N., Henry M., Schiller I., Nahid P., Hopewell PC., Ramsay A., Pai M., Laal S. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 2009. 16:260–76.
Article6). Houghton RL., Lodes MJ., Dillon DC., Reynolds LD., Day CH., McNeill PD., Hendrickson RC., Skeiky YA., Sampaio DP., Badaro R., Lyashchenko KP., Reed SG. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2002. 9:883–91.
Article7). Zhang SL., Zhao JW., Sun ZQ., Yang EZ., Yan JH., Zhao Q., Zhang GL., Zhang HM., Qi YM., Wang HH., Sun QW. Development and evaluation of a novel multiple-antigen ELISA for serodiagnosis of tuberculosis. Tuberculosis. 2009. 89:278–84.
Article8). Shin AR., Shin SJ., Lee KS., Eom SH., Lee SS., Lee BS., Lee JS., Cho SN., Kim HJ. Improved sensitivity of diagnosis of tuberculosis in patients in Korea via a cocktail enzyme-linked immunosorbent assay containing the abundantly expressed antigens of the K strain of Mycobacterium tuberculosis. Clin Vaccine Immunol. 2008. 15:1788–95.9). Malen H., Softeland T., Wiker HG. Antigen analysis of Mycobacterium tuberculosis H37Rv culture filtrate proteins. Scand J Immunol. 2008. 67:245–52.10). Ben Amor Y., Shashkina E., Johnson S., Bifani PJ., Kurepina N., Kreiswirth B., Bhattacharya S., Spencer J., Rendon A., Catanzaro A., Gennaro ML. Immunological characterization of novel secreted antigens of Mycobacterium tuberculosis. Scand J Immunol. 2005. 61:139–46.11). Laal S., Skeiky YA. Immune-based methods. In:. Cole ST, Eisenach KD, McMurray DN, Jacobs WR, editors. editors.Tuberculosis and the Tubercle Bacillus. Washington, D.C.: American Society for Microbiology Press;2005. p. 71–83.12). Yuan Y., Crane DD., Simpson RM., Zhu YQ., Hickey MJ., Sherman DR., Barry CE 3rd. The 16-kDa alpha-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci USA. 1998. 95:9578–83.13). Raja A., Uma Devi KR., Ramalingam B., Brennan PJ. Immunoglobulin G, A, and M responses in serum and circulating immune complexes elicited by the 16-kilodalton antigen of Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 2002. 9:308–12.14). Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–5.
Article15). Lee KS., Park JK., Lim JH., Kim SY., Shin AR., Yang CS., Oh JH., Kwon YM., Song CH., Jo EK., Kim HJ. Identification of proteins induced at hypoxic and low pH conditions in Mycobacterium tuberculosis H37Rv. J Bacteriol Virol. 2006. 36:59–68.16). Lee BY., Hefta SA., Brennan PJ. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992. 60:2066–74.17). Chua-Intra B., Wilkinson RJ., Ivanyi J. Selective T-cell recognition of the N-terminal peptide of GroES in tuberculosis. Infect Immun. 2002. 70:1645–7.
Article18). Camus JC., Pryor MJ., Medigue C., Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002. 148:2967–73.19). BahK YY., Kim SA., Kim JS., Euh HJ., Bai GH., Cho SN., Kim YS. Antigens secreted from Mycobacterium tuberculosis: identification by proteomics approach and test for diagnostic marker. Proteomics. 2004. 4:3299–307.20). Gregory RL. Microbial ribosomal vaccines. Rev Infect Dis. 1986. 8:208–17.
Article21). Rosenkrands I., Aagaard C., Weldingh K., Brock I., Dziegiel MH., Singh M., Hoff S., Ravn P., Andersen P. Identification of Rv0222 from RD4 as a novel serodiagnostic target for tuberculosis. Tuberculosis. 2008. 88:335–43.
Article22). Sartain MJ., Slayden RA., Singh KK., Laal S., Belisle JT. Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profiling. Mol Cell Proteomics. 2006. 5:2102–13.
Article23). Singh KK., Sharma N., Vargas D., Liu Z., Belisle JT., Potharaju V., Wanchu A., Behera D., Laal S. Peptides of a novel Mycobacterium tuberculosis-specific cell wall protein for immunodiagnosis of tuberculosis. J Infect Dis. 2009. 200:571–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of an ELISA kit for the Serodiagnosis of Pulmonary Tuberculosis by Using Mixed Antigens of Mycobacterium Tuberculosis
- Seroreactive Mycobacterial Proteins and Lipid in Cattle with Bovine Tuberculosis
- New Diagnostic Methods for Mycobacterium Tuberculosis Infection
- Diagnostic Significance of the Serologic Test Using Multiple Antigens of Mycobacterium Tuberculosis by ELISA
- Production and Characterization of Monoclonal Antibodies Reactive to PPD of Mycobacterium Tuberculosis