J Bacteriol Virol.
2009 Dec;39(4):317-327. 10.4167/jbv.2009.39.4.317.
Detection and Identification of the Spotted Fever Group Rickettsial Agents from Haemaphysalis Ticks in Jeju Island, Korea
- Affiliations
-
- 1Institute of Environmental Resource Research of Jeju Special Self-Governing Province, Jeju, Korea.
- 2Department of Microbiology, College of Medicine, Konkuk University, Seoul, Korea. wjjang@kku.ac.kr
- 3Department of Medical Entomology, Korea Center for Disease Control and Prevention, Seoul, Korea.
- 4Division of Biosafety Evaluation and Control, Korea Center for Disease Control and Prevention, Seoul, Korea.
- KMID: 2168543
- DOI: http://doi.org/10.4167/jbv.2009.39.4.317
Abstract
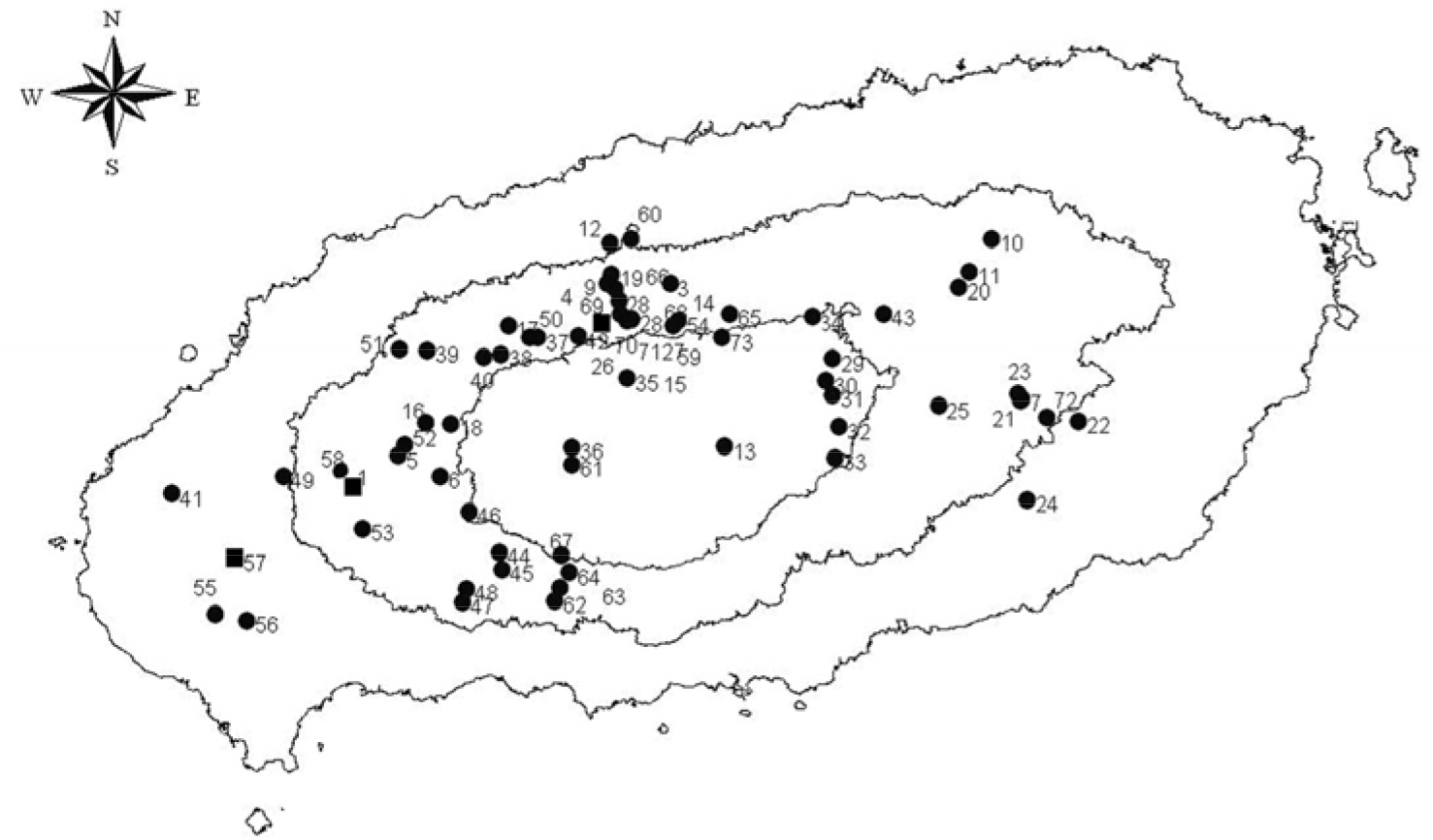
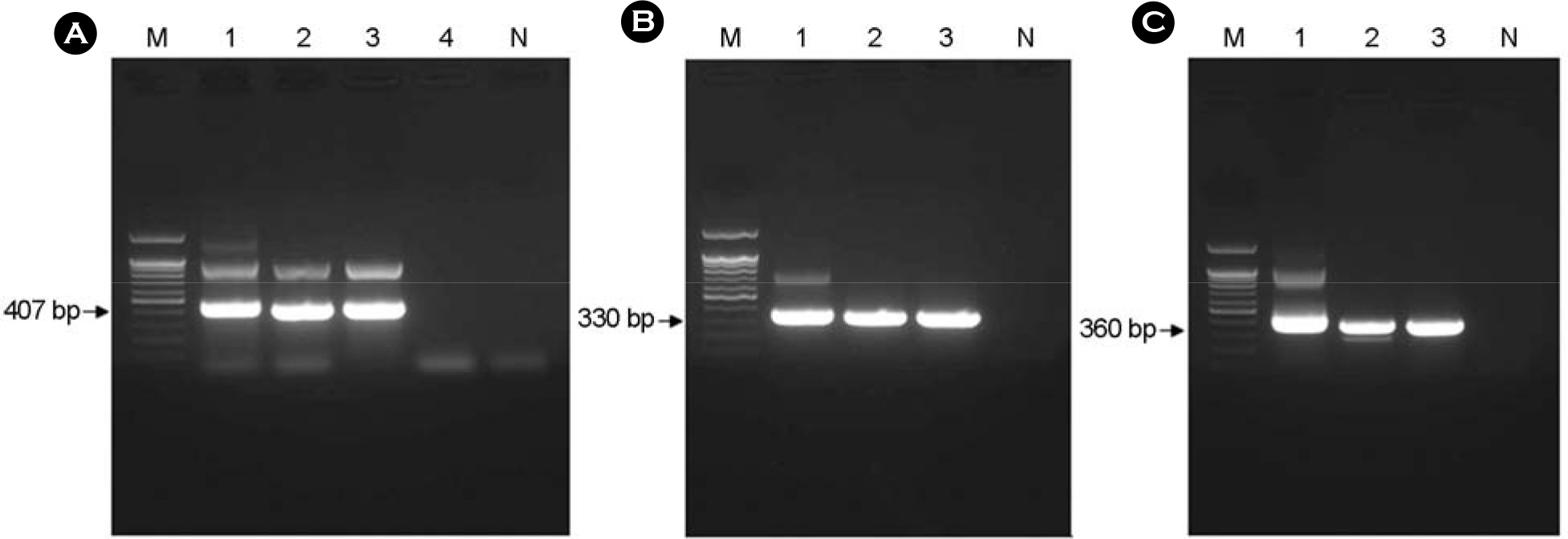
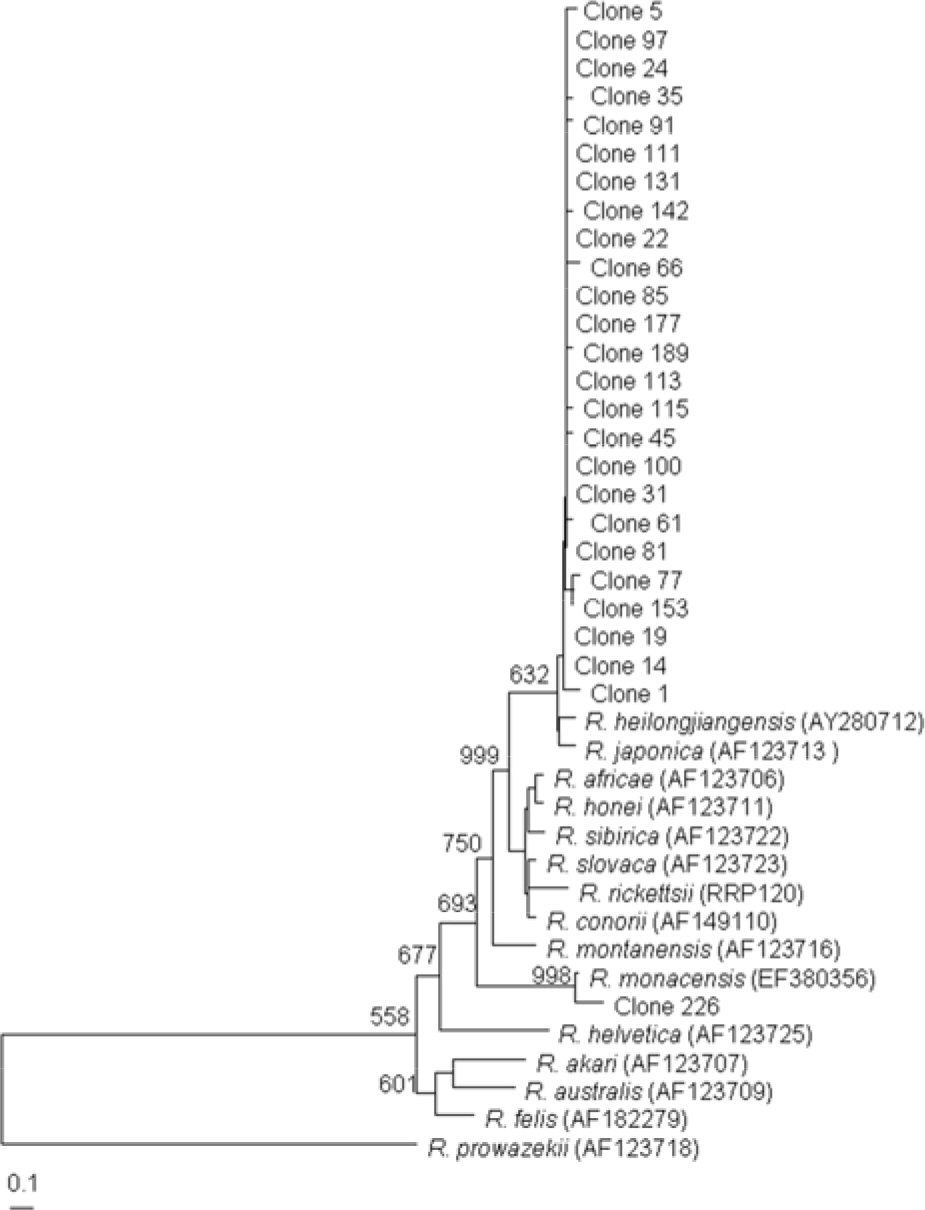
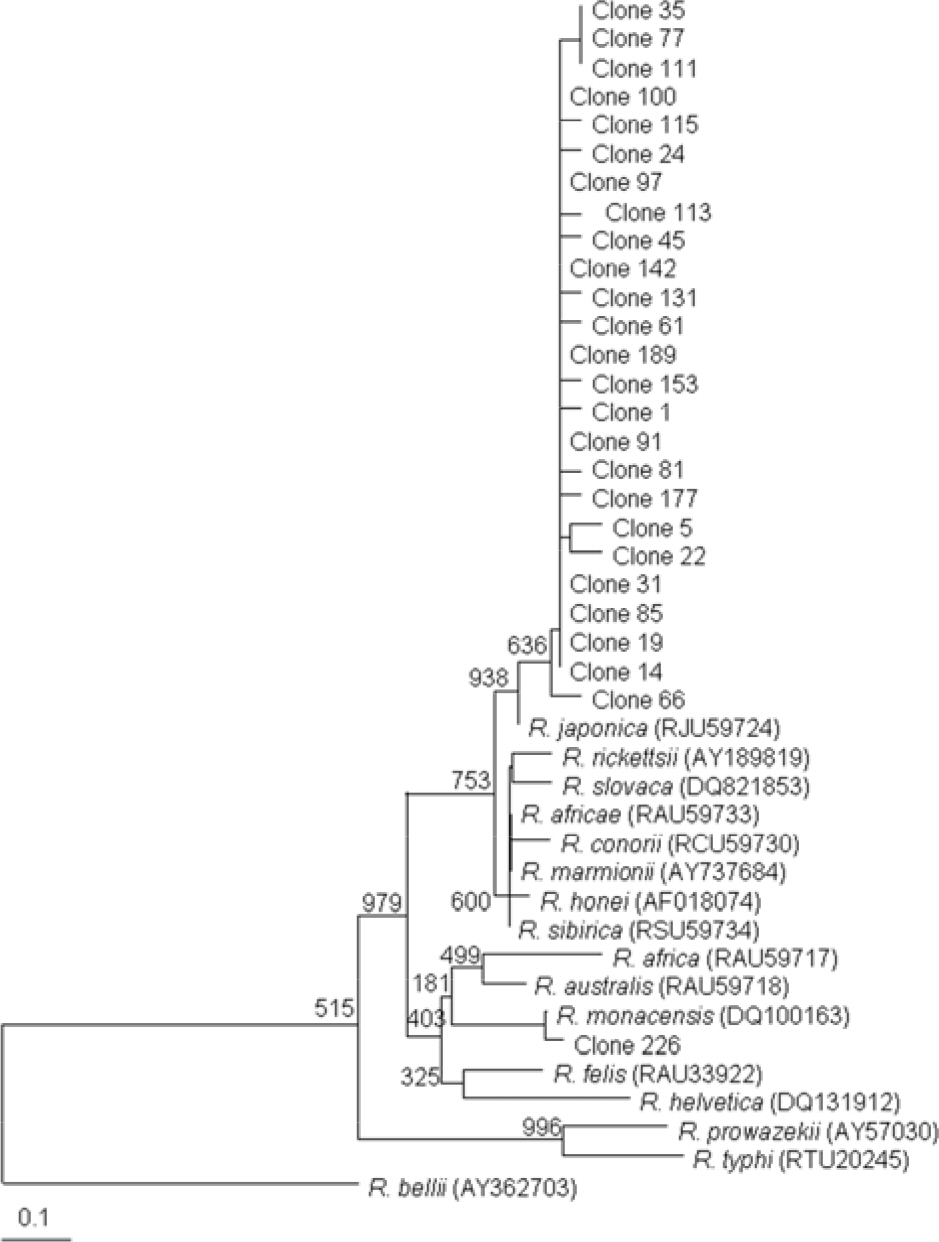
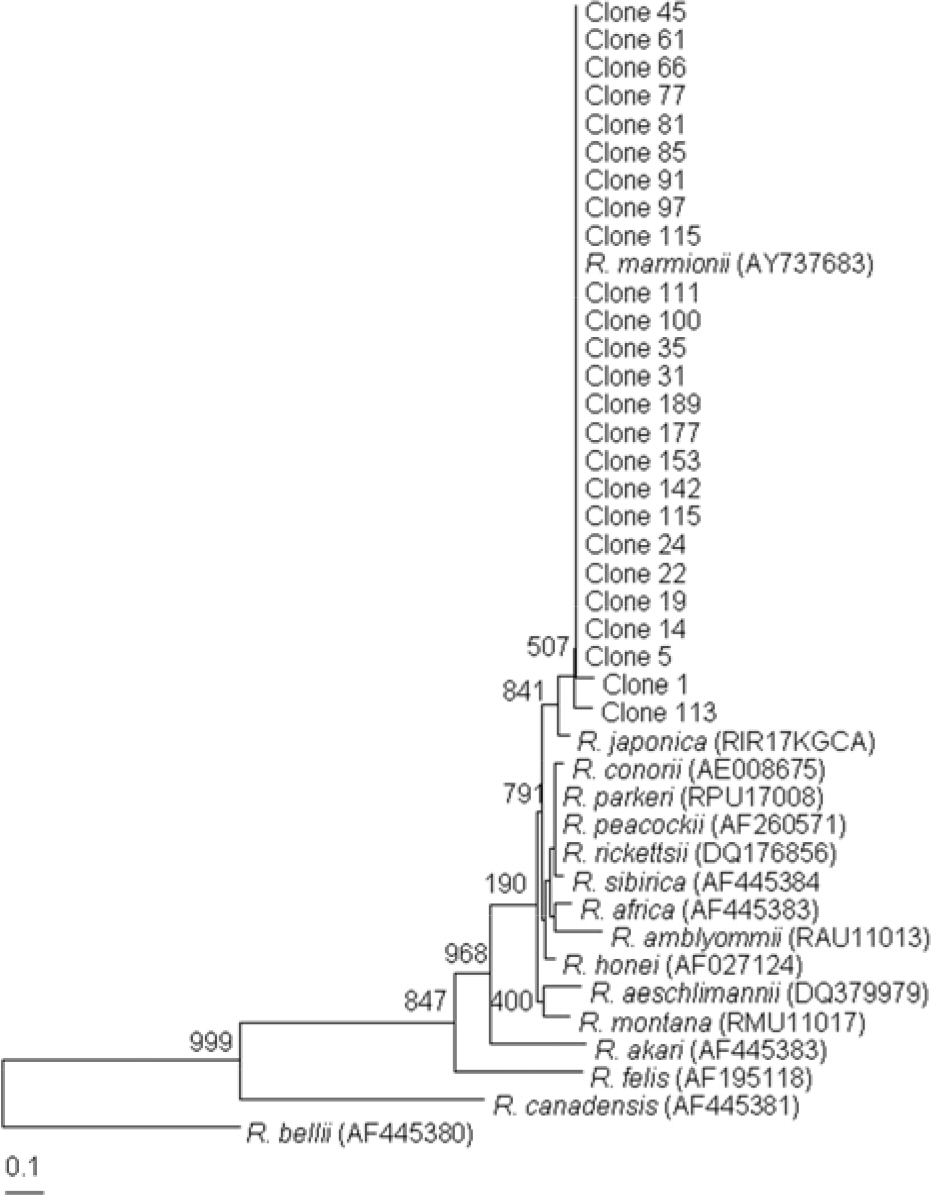
- This study investigated the presence of nucleic acids of various Rickettsial agents in ticks collected in Jeju Island, Korea from June 2007 to August 2008, through the nested polymerase chain reaction (PCR) and sequencing analysis of partial citrate synthase (gltA), Rickettsial outer membrane protein B (ompB), and 17-kDa genes. Examination of the 1,584 ticks showed that the subspecies distribution of Haemaphysalis longicornis was 99.81% (n=1,581) and H. flava was 0.19% (n=3). A total 224 out of 250 pools from one to 15 ticks were found to be positive in ompB-PCR assay (minimal infection rate 141 ticks/1,000 tested). From the positive samples, 26 were analyzed by gltA- and 17-kDa-PCR assays. The nucleotide sequences of the ompB- and gltA-PCR products showed a high degree of similarity with those of the Rickettsia japonica (98.7~99.2% and 98.7~99.3%, n=25) and R. monacensis (99% and 99.7%, n=1). However, analysis of the nucleotide sequences of the 17-kDa-PCR amplicons showed that the sequences of the 25 PCR amplicons were more close to R. marmionii (99.4~100%) than R. japonica (98.6~99.1%). These findings suggest that various rickettsial diseases could be transmitted via the bite of tick vectors in Jeju Island, Korea.
Keyword
MeSH Terms
Figure
Reference
-
1). Dumler JS., Barbet AF., Bekker CP., Dasch GA., Palmer GH., Ray SC., Rikihisa Y., Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001. 51:2145–65.2). Anacker RL., Pickens EG., Lackman DB. Details of the ultrastructure of Rickettsia prowazekii grown in the chick yolk sac. J Bacteriol. 1967. 94:260–2.3). Pang H., Winkler HH. Analysis of the peptidoglycan of Rickettsia prowazekii. J Bacteriol. 1994. 176:923–6.4). Silverman DJ., Wisseman CL Jr. Comparative ultrastructural study on the cell envelopes of Rickettsia prowazekii, Rickettsia rickettsii, and Rickettsia tsutsugamushi. Infect Immun. 1978. 21:1020–3.5). Drancourt M., Raoult D. Taxonomic position of the rickettsiae: current knowledge. FEMS Microbiol Rev. 1994. 13:13–24.
Article6). Anderson BE., Tzianabos T. Comparative sequence analysis of a genus-common rickettsial antigen gene. J Bacteriol. 1989. 171:5199–201.
Article7). Fournier PE., Raoult D. Current knowledge on phylogeny and taxonomy of Rickettsia spp. Ann N Y Acad Sci. 2009. 1166:1–11.8). Roux V., Raoult D. Phylogenetic analysis of the members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol. 2000. 50:1449–55.9). Sekeyová Z., Fournier PE., Rehácek J., Raoult D. Characterization of a new spotted fever group Rickettsia detected in Ixodes ricinus (Acari: Ixodidae) collected in Slovakia. J Med Entomol. 2000. 37:707–13.10). Williams SG., Sacci JB Jr., Schriefer ME., Andersen EM., Fujioka KK., Sorvillo FJ., Barr AR., Azad AF. Typhus and typhus like rickettsiae associated with opossums and their fleas in Los Angeles County, California. J Clin Microbiol. 1992. 30:1758–62.11). Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001. 32:897–928.
Article12). Raoult D., Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997. 10:694–719.
Article13). 김관천, 이진종, 김종선, 조영관. 위생곤충학. 파주: 신광문화사,. 2007.14). 이순형, 채종일, 홍성태. 임상기생충학 개요. 서울: 고려과학. 1996.15). 한국동물분류학회. 한국동물명집. 서울: 아카데미서적. 1997.16). Noh YT. A Taxonomical Study on Ticks in Korea. 한국생물 상에 관한 연구. 과학기술처 R-72-82. 1972. 140–60.17). Kim JE., Park HJ., Lee JY., Cho BK., Lee IY., Lee WK., Koh CJ. Three cases of tick bites by Haemophysalis longicornis. Korean J Dermatol. 2003. 41:1198–201.18). Roh DK., Jang IG., Cho BK., Son SJ., Lee IY., Lee WK. Tick bite by Haemophysalis longicornis Neumann: Laboratory observation of the causative tick. Korean J Dermatol. 1999. 37:631–6.19). Yoon SY., Kim KH., Suhr KB., Cho BK., Nam IH., Lee WK., Lee JH., Park JK. A case of tick bite caused by Haemophysalis flava. Korean J Dermatol. 1996. 34:326–30.20). Unsworth NB., Stenos J., Graves SR., Faa AG., Cox GE., Dyer JR., Boutlis CS., Lane AM., Shaw MD., Robson J., Nissen MD. Flinders Island spotted fever rickettsioses caused by “marmionii” strain of Rickettsia honei, Eastern Australia. Emerg Infect Dis. 2007. 13:566–73.21). Yamaguti N., Tipton VJ., Keegan H., Toshioka S. Ticks of Japan, Korea, and the Ryukyu islands. Brigham Young University Science Bulletin. 1971. 15:1–126.22). Choi YJ., Lee SH., Park KH., Koh YS., Lee KH., Baik HS., Choi MS., Kim IS., Jang WJ. Evaluation of PCR-based assay for diagnosis of spotted fever group rickettsiosis in human serum samples. Clin Diagn Lab Immunol. 2005. 12:759–63.
Article23). Choi YJ., Jang WJ., Kim JH., Ryu JS., Lee SH., Park KH., Paik HS., Koh YS., Choi MS., Kim IS. Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis. 2005. 11:237–44.
Article24). Regnery RL., Spruill CL., Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspeecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991. 173:1576–89.25). Leitner M., Yitzhaki S., Rzotkiewicz S., Keysary A. Polymerase chain reaction-based diagnosis of Mediterranean spotted fever in serum and tissue samples. Am J Trop Med Hyg. 2002. 67:166–9.
Article26). Rolain JM., Maurin M., Vestris G., Raoult D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother. 1998. 42:1537–41.27). Gaywee J., Sunyakumthorn P., Rodkvamtook W., Ruang-areerate T., Mason CJ., Sirisopana N. Human infection with Rickettsia sp. related to R. japonica, Thailand. Emerg Infect Dis. 2007. 13:671–3.28). Mahara F., Koga K., Sawada S., Taniguchi T., Shigemi F., Suto T., Tsuboi Y., Ooya A., Koyama H., Uchiyama T. The first report of the rickettsial infections of spotted fever group in Japan: three clinical cases. Kansenshogaku Zasshi. 1985. 59:1165–71.
Article29). Lee JH., Park HS., Jung KD., Jang WJ., Koh ES., Kang SS., Lee IY., Lee WJ., Kim BJ., Kook YH., Park KH., Lee SH. Identification of the spotted fever group rickettsiae detected from Haemaphysalis longicornis in Korea. Microbiol Immunol. 2003. 47:301–4.30). Chung MH., Lee SH., Kim MJ., Lee JH., Kim ES., Lee JS., Kim MK., Park MY., Kang JS. Japanese spotted fever, South Korea. Emerg Infect Dis. 2006. 12:1122–4.
Article31). Weller SJ., Baldridge GD., Munderloh UG., Noda H., Simser J., Kurtti TJ. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol. 1998. 36:1305–17.32). Dib L., Bitam I., Bensouilah M., Parola P., Raoult D. First description of Rickettsia monacensis in Ixodes ricinus in Algeria. Clin Microbiol Infect. 2009. May 27 [Epub ahead of print].33). Jado I., Oteo JA., Aldámiz M., Gil H., Escudero R., Ibarra V., Portu J., Portillo A., Lezaun MJ., García-Amil C., Rodríguez-Moreno I., Anda P. Rickettsia monacensis and human disease, Spain. Emerg Infect Dis. 2007. 13:1405–7.34). Unsworth NB., Stenos J., Faa AG., Graves SR. Three rickettsioses, Darnley Island, Australia. Emerg Infect Dis. 2007. 13:1105–7.
Article35). Graves S., Stenos J. Rickettsioses in Australia. Ann N Y Acad Sci. 2009. 1166:151–5.
Article36). Jang WJ., Kim JH., Choi YJ., Jung KD., Kim YG., Lee SH., Choi MS., Kim IS., Walker DH., Park KH. First serologic evidence of human spotted fever group rickettsiosis in Korea. J Clin Microbiol. 2004. 42:2310–3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of Spotted Fever Group Rickettsia from Haemaphysalis Ticks in Chungju Province
- Coinfection of Severe Fever With Thrombocytopenia Syndrome Virus and Coxiella burnetii in Developmental Stage of Hard Ticks in Subtropical Region of Korea
- Rapid Identification of Rickettsiae using the Real-Time PCR
- Molecular Detection of Toxoplasma Gondii in Haemaphysalis Ticks in Korea
- No Detection of Severe Fever with Thrombocytopenia Syndrome Virus from Ixodid Ticks Collected in Seoul