Genotype, Coagulase Type and Antimicrobial Susceptibility of Methicillin-Resistant Staphylococcus aureus Isolated from Dermatology Patients and Healthy Individuals in Korea
- Affiliations
-
- 1Department of Clinical Laboratory Science, Wonkwang Health Science University, Iksan, Korea. smkim1211@hanmail.net
- 2Department of the Dermatology, Wonkwang University Hospital, Iksan, Korea.
- 3Vestibulocochlear Research Center & Department of Microbiology, College of Medicine, Wonkwang University, Iksan, Korea.
- 4Department of Clinical Laboratory Science, Catholic University of Pusan, Busan, Korea.
- 5Department of Internal Medicine, Division of Infectious Diseases, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 6College of Pharmacy and Wonkwang-Oriental Medicines Research Institute, Wonkwang University, Iksan, Korea.
- 7Department of Clinical Laboratory Science, Dong-eui Institute of Technology University, Busan, Korea.
- 8Department of Biology, Sunchon National University, Sunchon, Korea.
- 9Division of Life-Environment, College of Life Science and Natural Resources, Wonkwang University, Iksan, Korea.
- 10Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2168542
- DOI: http://doi.org/10.4167/jbv.2009.39.4.307
Abstract
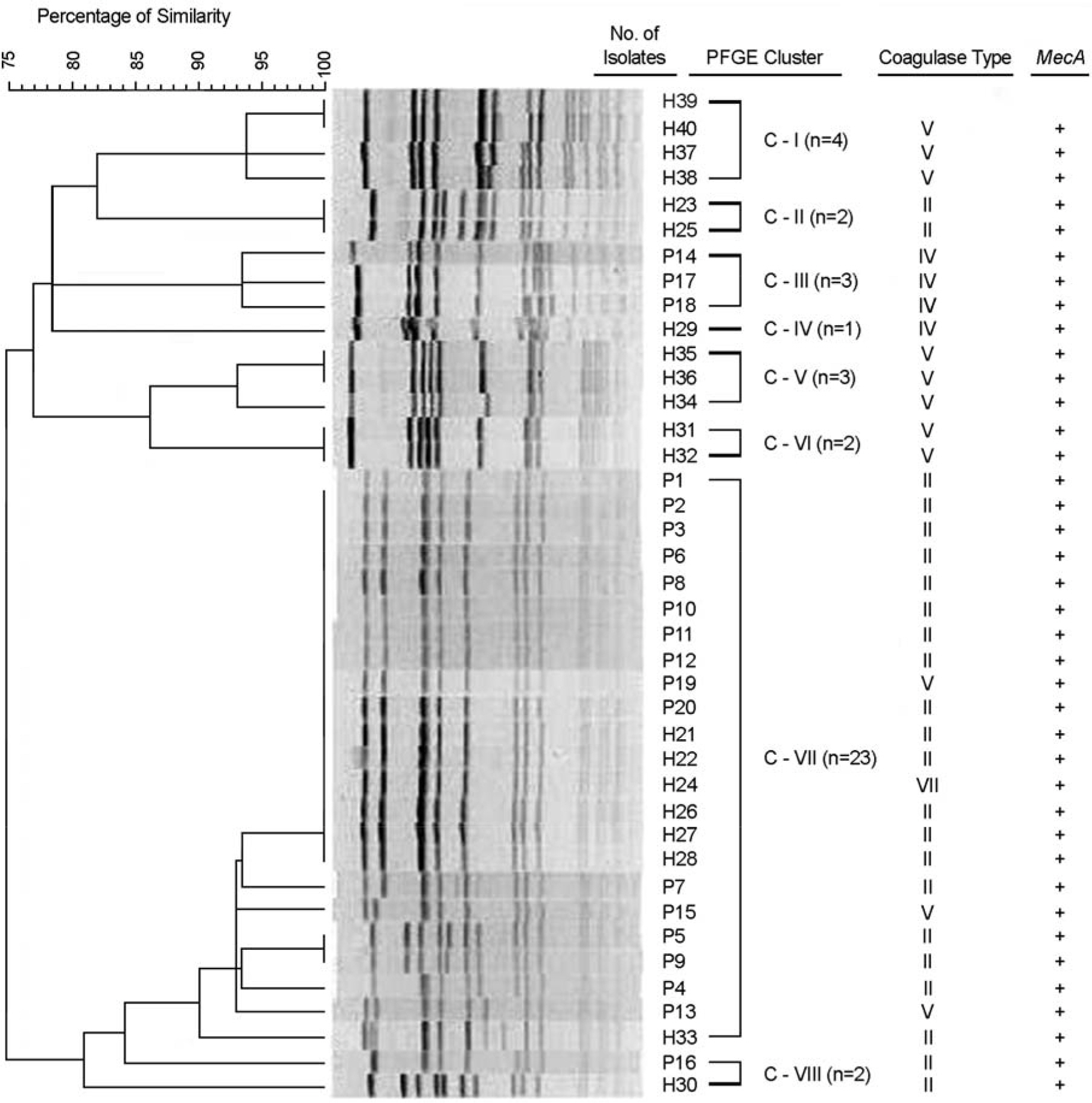
- Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most prevalent dermatology pathogens in hospitals and increasingly recognized in communities. We determined PFGE pattern of SmaI-restricted genomic DNA, coagulase type, and antimicrobial susceptibility of MRSA isolated in 2008 from dermatology inpatients and healthy hospital employees in A Hospital and from primary school children in Iksan city, Korea. Overall, the isolation rate of MRSA was 3.8% from the 788 normal persons: 4.9% from hospital employees and 1.1% from primary school children. MRSA was isolated in six of 13 (46.2%) family members of four school children with MRSA. The most prevalent coagulase serotype was II from patients and V from healthy individuals. Ten of twenty and six of twenty MRSA isolates from patients and from healthy personnel, respectively, had identical PFGE patterns, suggesting that these are originated from identical clones. Against MRSA from patients, only vancomycin was the most active (MIC range < or =2 microg/ml), whereas the resistance rates were 35% to rifampin and 65% to mupirocin. The resistance rates of patient isolates were > or =90% to amikacin, clindamycin, ciprofloxacin, erythromycin, fusidic acid, gentamicin and tetracycline. In conclusion, the MRSA carriage rates of healthy hospital workers were relatively high, 2.3~7.7%, depending on groups. Family members of a few primary school children with MRSA showed a high carriage rate, suggesting that intrafamily transmission occurred. MRSAs isolated from dermatology inpatients were relatively more resistant to various antimicrobial agents, including mupirocin, but all isolates were susceptibility to vancomycin.
Keyword
MeSH Terms
-
Amikacin
Anti-Infective Agents
Child
Ciprofloxacin
Clindamycin
Clone Cells
Coagulase
Dermatology
DNA
Erythromycin
Fusidic Acid
Genotype
Gentamicins
Humans
Inpatients
Korea
Methicillin Resistance
Methicillin-Resistant Staphylococcus aureus
Mupirocin
Rifampin
Tetracycline
Vancomycin
Natural Resources
Amikacin
Anti-Infective Agents
Ciprofloxacin
Clindamycin
Coagulase
DNA
Erythromycin
Fusidic Acid
Gentamicins
Mupirocin
Rifampin
Tetracycline
Vancomycin
Figure
Cited by 3 articles
-
Application of Infrequent-Restriction-Site Polymerase Reaction (IRS-PCR) to the Molecular Epidemiologic Analysis of Methicillin Resistant Staphylococcus aureus (MRSA)
Na-Young Shin, Jin-Hong Yoo, Chulmin Park, Dong-Gun Lee, Su-Mi Choi, Jae-Cheol Kwon, Si-Hyun Kim, Sun-Hee Park, Jung-Hyun Choi
Infect Chemother. 2011;43(5):396-405. doi: 10.3947/ic.2011.43.5.396.The Prevalence, Genotype and Antimicrobial Susceptibility of High- and Low-Level Mupirocin Resistant Methicillin-Resistant Staphylococcus aureus
Se Young Park, Shin Moo Kim, Seok Don Park
Ann Dermatol. 2012;24(1):32-38. doi: 10.5021/ad.2012.24.1.32.The Prevalence, Genotype and Antimicrobial Susceptibility of High- and Low-Level Mupirocin Resistant Methicillin-Resistant Staphylococcus aureus
Se Young Park, Shin Moo Kim, Seok Don Park
Ann Dermatol. 2012;24(1):32-38. doi: 10.5021/ad.2012.24.1.32.
Reference
-
1). Jevons MP. “Celbenin”-resistant staphylococci. Br Med J. 1961. 124:124–5.2). Saravolatz LD., Markowitz N., Arking L., Pohlod D., Fisher E. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982. 96:11–6.3). Hussain FM., Boyle-Vavra S., Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr Infect Dis J. 2001. 20:763–7.4). Suggs AH., Maranan MC., Boyle-Vavra S., Daum RS. Methicillin-resistant and borderline methicillin-resistant asymptomatic Staphylococcus aureus colonization in children without identifiable risk factors. Pediatr Infect Dis J. 1999. 18:410–4.5). Miller LG., Perdreau-Remington F., Rieg G., Mehdi S., Perlroth J., Bayer AS., Tang AW., Phung TO., Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005. 352:1445–53.6). Chong Y., Lee K. Present situation of antimicrobial resistance in Korea. J Infect Chemother. 2000. 6:189–95.
Article7). Kim JM., Park ES., Jeong JS., Kim KM., Kim JM., Oh HS., Yoon SW., Chang HS., Chang KH., Lee SI., Lee MS., Song JH., Kang MW., Park SC., Choe KW., Pai CH. Multicenter surveillance study for nosocomial infections in major hospitals in Korea. Nosocomial Infection Surveillance Committee of the Korean Society for Nosocomial Infection Control. Am J Infect Control. 2000. 28:454–8.8). Kim HB., Sa CM., Yoo J., Kim BS., Yun OJ., Yoon HR., Lee YS. Antibiotic resistance patterns of Staphylococcus aureus isolated from the patients admitted to non-tertiary hospitals. Korean J Infect Dis. 2000. 32:259–63.9). Tenover FC., Arbeit R., Archer G., Biddle J., Byrne S., Goering R., Hancock G., Hébert GA., B Hill., Hollis R. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J Clin Microbiol. 1994. 32:407–15.10). Aarestrup FM., Wegener HC., Rosdahl VT. Evaluation of phenotypic and genotypic methods for epidemiological typing of Staphylococcus aureus isolates from bovine mastitis in Denmark. Vet Microbiol. 1995. 45:139–50.11). Prevost G., Jaulhac B., Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992. 30:967–73.12). Saulnier P., Bourneix C., Prevost G., Andremont A. Random amplified polymorphic DNA assay is less discriminant than pulsed-field gel electrophoresis for typing strains of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993. 31:982–5.13). Ichiyama S., Ohta M., Shimokata K., Kato N., Takeuchi J. Genomic DNA fingerprinting by pulsed-field gel electrophoresis as an epidemiological marker for study of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1991. 29:2690–5.14). Yokoyama TS. Study on mec gene in methicillin-resistant Staphylococci. Kansenshogaku Zasshi. 1993. 67:1203–10.15). Lee HK., Lee EJ., Pahk YJ., Kim BK., Kang MW., Shim SI. Relationship between the level of methicillin resistance and mecA, mecI, femA, genes in Staphylococci. Korean J Infec Dis. 1998. 30:36–44.16). Hwang SM., Kim TU. Changes in coagulase serotype of Staphylococcus aureus isolates in Busan, 1994~2005. Korean J Microbiol. 2007. 43:346–50.17). Hwang SM., Seki K., Sakurata J., Ogasawara M., Murai M., Ohmayu S., Kurosaka K., Masuda S. Improved methods for detection and serotyping of coagulase from Staphylococcus aureus. Microbiol Immunol. 1989. 33:175–82.18). Murchan S., Kaufmann ME., Deplano A., de Ryck R., Struelens M., Zinn CE., Fussing V., Salmenlinna S., Vuopio-Varkila J., El Solh N., Cuny C., Witte W., Tassios PT., Legakis N., van Leeuwen W., van Belkum A., Vindel A., Laconcha I., Garaizar J., Haeggman S., Olsson-Liljequist B., Ransjo U., Coombes G., Cookson B. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J Clin Microbiol. 2003. 41:1574–85.19). CLSI. Performance standards for microbial susceptibility testing 19th informational supplement. M100-S19. CLSI. 2009. Wayne Pa.20). MacGowan AP., Wise R. Establishing MIC breakpoints and the interpretation of in vitro susceptibility tests. J Antimicrob Chemother. 2001. 48:17–28.21). Hisata K., Kuwahara-Arai K., Yamanoto M., Ito T., Nakatomi Y., Cui L., Baba T., Terasawa M., Sotozono C., Kinoshita S., Yamashiro Y., Hiramatsu K. Dissemination of methicillin-resistant Staphylococci among healthy Japanese children. J Clin Microbiol. 2005. 43:3364–72.22). Garner JS., Jarvis WR., Emori TG., Horan TC., Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988. 16:128–40.
Article23). Stacey AR., Endersby KE., Chan PC., Marples RR. An outbreak of methicillin resistant Staphylococcus aureus infection in a rugby football team. Br J Sports Med. 1998. 32:153–4.24). Lindenmayer JM., Schoenfeld S., O'Grady R., Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community. Arch Intern Med. 1998. 158:895–9.25). CDC. methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison-Mississippi, 2000. Morp Mortal Wkly Rep. 2001. 50:919–22.26). Jones TF., Kellum ME., Porter SS., Bell M., Schaffner W. An outbreak of community-acquired foodborne illness caused by methicillin-resistant Staphylococcus aureus. Emerg Infect Dis. 2002. 8:82–4.27). Hisata K., Kuwahara-Arai K., Yamanoto M., Ito T., Nakatomi Y., Cui L., Baba T., Terasawa M., Sotozono C., Kinoshita S., Yamashiro Y., Hiramatsu K. Dissemination of Methicillin-resistant Staphylococci among healthy Japanese children. J Clin Microbiol. 2005. 43:3364–72.28). Hussain FM., Boyle-Vavra S., Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus colonization in healthy children attending an outpatient pediatric clinic. Pediatr Infect Dis J. 2001. 20:763–7.29). Suggs AH., Maranan MC., Boyle-Vavra S., Daum RS. Methicillin-resistant and borderline methicillin-resistant asymptomatic Staphylococcus aureus colonization in children without identifiable risk factors. Pediatr Infect Dis J. 1999. 18:410–4.30). Kim SM., Song NK., Shin SH., Chung JO., Lee GS., Kim YH., Oh JS., Cha CD., Moon SE., Kim KJ., Shim ES., Kim EC., Seong CN., Chong Y. Nasal carriage of MRSA among healthy individual and detection of mecA and femA gene methicillin-resistant Staphylococcus aureus. Korean J Clin Lab Sci. 1999. 31:91–104.31). Seong HK., Bae YS., Kim YH. The epidemiological of nasal colonization of methicillin-resistant Staphylococcus aureus in patients and doctors. J Exp Biomed Sci. 2004. 10:309–15.32). Moriwaki T. Genotyping of methicillin-resistant Staphylococcus aureus isolated from inpatients and medical workers in orthopedics ward. Kansenshogaku Zasshi. 2003. 77:1058–66.33). Nakahara S., Kawayama T., Yokoyama T., Akiyoshi H., Okubo Y., Honda J., Tokunaga N., Ichikawa Y., Oizumi K., Kajimura K. Antibiotics susceptibilities and other biological properties of methicillin-resistant Staphylococcus aureus: Observation in the last 2 years in our university hospital. Kansenshogaku Zasshi. 1994. 68:339–45.34). Lee MS., Chong Y. Characteristics of methicillin-resistant Staphylococcus aureus isolated from wounds in Korean patients. J Infect Chemother. 1996. 2:130–35.35). Kim EC., Jung HJ., Oh MD., Lee HJ., Oh HS., Choe KW. Epidemiological typing of methicillin-resistant Staphylococcus aureus outbreak isolates by pulsed field gel electrophoresis and antibiogram. Yonsei Med J. 1998. 39:587–94.36). Weber JT. Community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2005. 41:S269–72.37). Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001. 7:178–82.38). Afset JE., Maeland JA. Susceptibility of skin and soft-tissue isolates of Staphylococcus aureus and Streptococcus pyogenes to topical antibiotics: indications of clonal spread of fusidic acid-resistant Staphylococcus aureus. Scand J Infect Dis. 2003. 35:84–9.39). Larsen AR., Skov RL., Jarlier V., Henriksen AS. Epidemiological differences between the UK and Ireland versus France in Staphylococcus aureus isolates resistant to fusidic acid from community-acquired skin and soft tissue infections. J Antimicrob Chemother. 2008. 61:589–94.40). Ravenscroft JC., Layton AM., Eady EA., Murtagh MS., Coates P., Walker M., Cove JH. Short-term effects of topical fusidic acid or mupirocin on the prevalence of fusidic acid resistant (FusR) Staphylococcus aureus in atopic eczema. Br J Dermatol. 2003. 148:1010–7.41). Parras F., Guerrero MC., Bouza E., Blazquez MJ., Moreno S., Menarguez MC., Cercenado E. Comparative study of mupirocin and oral co-trimoxazole plus topical fusidic acid in eradication of nasal carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1995. 39:175–9.42). Schmitz FJ., Jones ME. Antibiotics for treatment of infections caused by MRSA and elimination of MRSA carriage. What are the choices? Int J Antimicrob Agents. 1997. 9:1–19.43). Rahman M., Noble WC., Cookson B. Mupirocin resistant Staphylococcus aureus. Lancet. ii:1987. 387–8.44). Yun HJ. Lee SW, Yoon GM, Kim SY, Choi S, Lee YS, Choi EC, Kim S. Prevalence and mechanisms of low-and high-level mupirocin resistance in staphylococci isolated from a Korean hospital. J Antimicrob Chemother. 2003. 51:619–23.45). Yoo JI., Shin ES., Cha JO., Lee JK., Jung YH., Lee KM., Kim BS., Lee YS. Clonal dissemination and mupA gene polymorphism of mupirocin-resistant Staphylococcus aureus isolates from long-term-care facilities in South Korea. Antimicrob Agents Chemother. 2006. 50:365–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation Between Staphylococcal Cassette Chromosome mec Type and Coagulase Serotype of Methicillin-Resistant Staphylococcus aureus
- Correlation between Sau1 Restriction and Modification Complex Type and Coagulase Serotype or SCCmec Type of Staphylococcus aureus
- Restriction Endonuclease Analysis of Plasmids and Antimicrobial Resistance Pattern of Staphylococcus Aureus and S. Epidermidis Isolated from Clinical Specimens
- Comparison of pathogens and antimicrobial susceptibility of Staphylococcus aureus isolated from conventional and robotic milking herds
- Detection of Multidrug Resistant Patterns and Associated - genes of Methicillin Resistant Staphylococcus aureus ( MRSA ) Isolated from Clinical Specimens