Allergy Asthma Respir Dis.
2015 Mar;3(2):99-104. 10.4168/aard.2015.3.2.99.
Plasma secreted phospholipase A2 in asthmatic children: correlation with leptin levels and exercise induced bronchoconstriction
- Affiliations
-
- 1Department of Pediatrics, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea. paviola7@hanmail.net
- 2Department of Pediatrics, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2168500
- DOI: http://doi.org/10.4168/aard.2015.3.2.99
Abstract
- PURPOSE
Dysregulated cysteinyl leukotriene (CysLT) synthesis is prominent in exercise-induced bronchoconstriction (EIB). Secreted phospholipase A2 (sPLA2) plays a key regulatory role in the biosynthesis of CysLTs. We previously found that serum leptin levels correlate with (EIB) in children with asthma. The aim of this study was to address the relationship between plasma sPLA2/leptin levels and EIB.
METHODS
Sixty-seven prepubertal children between the ages of 6 and 10 years were included in the study. They were asthmatics with EIB (n=25), asthmatics without EIB (n=21), and healthy subjects (n=21). We measured the plasma sPLA2 and leptin levels. We also performed pulmonary function tests at baseline, after bronchodilator inhalation, and after exercise.
RESULTS
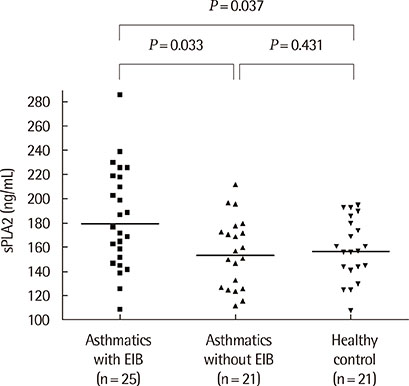
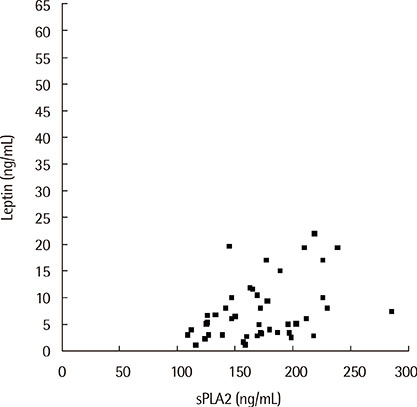
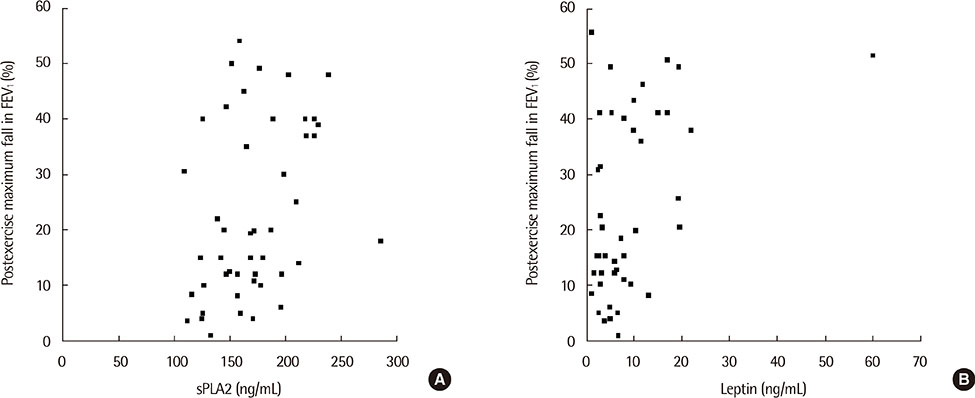
The sPLA2 and leptin levels were significantly higher in asthmatics with EIB than in those without and control subjects. In addition, sPLA2 levels were significantly correlated with body mass index (Speraman correlation coefficient r=0.343, P=0.023) and leptin levels (partial correlation coefficient r=318, P=0.033). The maximum decrease in % forced expiratory volume in 1 second after exercise was significantly correlated with both PLA2 levels (r=0.301, P=0.041) and leptin levels (r=0.346, P=0.018).
CONCLUSION
The sPLA2 and leptin levels were significantly higher in asthmatics with EIB than in asthmatics without EIB and control subjects. In addition, sPLA2 levels were significantly correlated with leptin levels and EIB in asthmatic children.
MeSH Terms
Figure
Reference
-
1. Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997; 155:421–425.
Article2. Chilton FH, Averill FJ, Hubbard WC, Fonteh AN, Triggiani M, Liu MC. Antigen-induced generation of lyso-phospholipids in human airways. J Exp Med. 1996; 183:2235–2245.
Article3. Stadel JM, Hoyle K, Naclerio RM, Roshak A, Chilton FH. Characterization of phospholipase A2 from human nasal lavage. Am J Respir Cell Mol Biol. 1994; 11:108–113.
Article4. Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR Jr. Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2007; 176:1072–1078.
Article5. Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011; 107:14–21.
Article6. Baek HS, Choi JH, Oh JW, Lee HB. Leptin and urinary leukotriene E4 and 9α,11β-prostaglandin F2 release after exercise challenge. Ann Allergy Asthma Immunol. 2013; 111:112–117.
Article7. Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004; 287:L497–L502.8. Misso NL, Petrovic N, Grove C, Celenza A, Brooks-Wildhaber J, Thompson PJ. Plasma phospholipase A2 activity in patients with asthma: association with body mass index and cholesterol concentration. Thorax. 2008; 63:21–26.
Article9. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178.
Article10. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–329.11. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, et al. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975; 56:323–327.
Article12. American Thoracic Society. European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.13. Hallstrand TS, Moody MW, Wurfel MM, Schwartz LB, Henderson WR Jr, Aitken ML. Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2005; 172:679–686.
Article14. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011; 29:415–445.
Article15. Hallstrand TS. New insights into pathogenesis of exercise-induced bronchoconstriction. Curr Opin Allergy Clin Immunol. 2012; 12:42–48.
Article16. Hallstrand TS, Moody MW, Aitken ML, Henderson WR Jr. Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005; 116:586–593.
Article17. Mito N, Kitada C, Hosoda T, Sato K. Effect of diet-induced obesity on ovalbumin-specific immune response in a murine asthma model. Metabolism. 2002; 51:1241–1246.
Article18. Back M, Sultan A, Ovchinnikova O, Hansson GK. 5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res. 2007; 100:946–949.19. Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, et al. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007; 176:650–658.
Article20. Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008; 121:1087–1093.
Article21. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005; 115:103–109.
Article22. Taildeman J, Perez-Novo CA, Rottiers I, Ferdinande L, Waeytens A, De Colvenaer V, et al. Human mast cells express leptin and leptin receptors. Histochem Cell Biol. 2009; 131:703–711.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exercise induced delayed bronchoconstriction in children with asthma
- Nitric Oxide Correlates with Exercise-Induced Bronchoconstriction in Asthmatic Children
- Exercise-Induced Bronchoconstriction
- Plasma Leptin Concentration in Patients with Chronic Renal Failure
- The Production of Phospholipase A2 in Different Types of Cultured Human Intervertebral Disc Cells