Electrolyte Blood Press.
2012 Dec;10(1):7-11. 10.5049/EBP.2012.10.1.7.
A New Technical Approach to Monitor the Cellular Physiology by Atomic Force Microscopy
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Kyung Hee University, Seoul, Korea. rulale@dreamwiz.com
- KMID: 2168388
- DOI: http://doi.org/10.5049/EBP.2012.10.1.7
Abstract
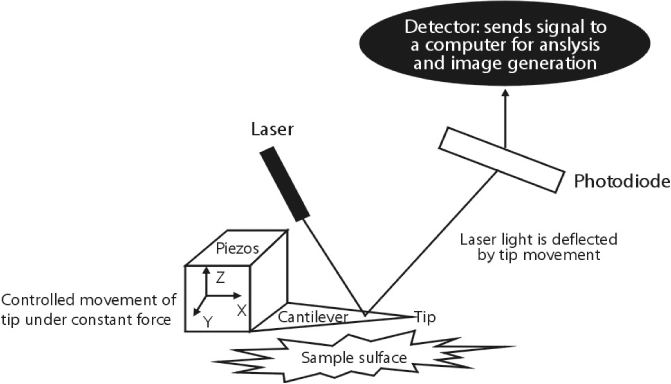
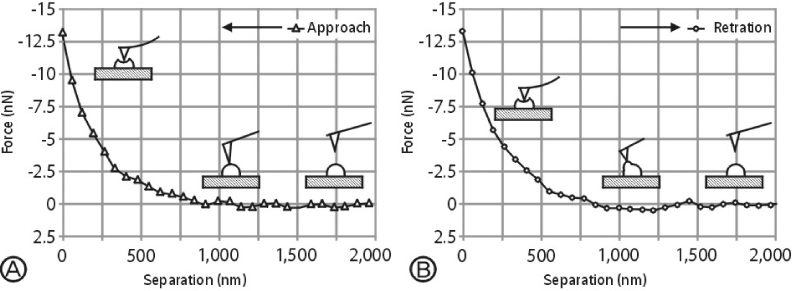
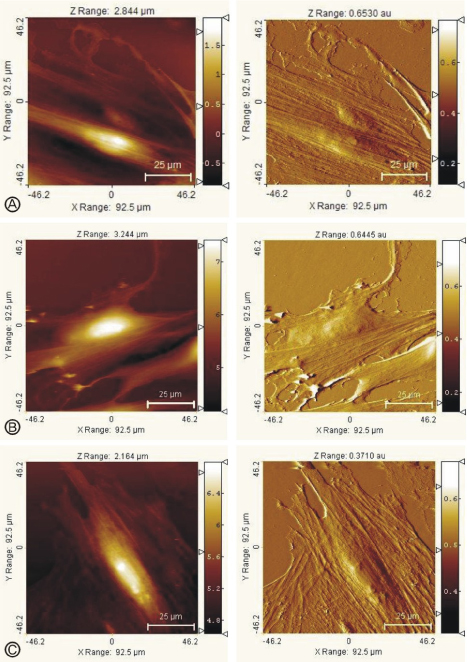
- Atomic force microscopy (AFM) has become an important medical and biological tool for non-invasive imaging and measuring the mechanical changes of cells since its invention by Binnig et al. AFM can be used to investigate the mechanical properties of cellular events in individual living cells on a nanoscale level. In addition, the dynamic cellular movements induced by biochemical activation of specific materials can be detected in real time with three dimensional resolution. Force measurement with the use of AFM has become the tool of choice to monitor the mechanical changes of variable cellular events. In addition, the AFM approach can be applied to measure cellular adhesion properties. Moreover, the information gathered from AFM is important to understanding the mechanisms related to cellular movement and mechanical regulation. This review will discuss recent contributions of AFM to cellular physiology with a focus on monitoring the effects of antihypertensive agents in kidney cells.
MeSH Terms
Figure
Reference
-
1. Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986. 56:930–933.
Article2. Volle CB, Ferguson MA, Aidala KE, et al. Quantitative changes in the elasticity and adhesive properties of escherichia coli zk1056 prey cells during predation by bdellovibrio bacteriovorus 109. Langmuir. 2008. 24:8102–8110.
Article3. Radmacher M, Tillamnn RW, Fritz M, et al. From molecules to cells: Imaging soft samples with the atomic force microscope. Science. 1992. 257:1900–1905.
Article4. Radmacher M, Fritz M, Kacher CM, et al. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys J. 1996. 70:556–567.
Article5. Schneider SW, Yano Y, Sumpio BE, et al. Rapid aldosterone-induced cell volume increase of endothelial cells measured by the atomic force microscope. Cell Biol Int. 1997. 21:759–768.
Article6. Oberleithner H. Aldosterone makes human endothelium stiff and vulnerable. Kidney Int. 2005. 67:1680–1682.
Article7. Oberleithner H, Riethmuller C, Ludwig T, et al. Aldosterone remodels human endothelium. Acta Physiol (Oxf). 2006. 187:305–312.
Article8. Kliche K, Kuhn M, Hillebrand U, et al. Direct aldosterone action on mouse cardiomyocytes detected with atomic force microscopy. Cell Physiol Biochem. 2006. 18:265–274.
Article9. Klahr S, Morrissey J. Angiotensin ii and gene expression in the kidney. Am J Kidney Dis. 1998. 31:171–176.
Article10. Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin ii and renal fibrosis. Hypertension. 2001. 38:635–638.
Article11. Lee GJ, Park EJ, Choi S, et al. Observation of angiotensin ii-induced changes in fixed and live mesangial cells by atomic force microscopy. Micron. 2010. 41:220–226.
Article12. Auger-Messier M, Turgeon ES, Leduc R, et al. The constitu-tively active n111g-at1 receptor for angiotensin ii modifies the morphology and cytoskeletal organization of hek-293 cells. Exp Cell Res. 2005. 308:188–195.
Article13. Cuerrier CM, Benoit M, Guillemette G, et al. Real-time monitoring of angiotensin ii-induced contractile response and cytoskeleton remodeling in individual cells by atomic force microscopy. Pflugers Arch. 2009. 457:1361–1372.
Article14. Hillebrand U, Lang D, Telgmann RG, et al. Nebivolol decreases endothelial cell stiffness via the estrogen receptor beta: A nanoimaging study. J Hypertens. 2009. 27:517–526.
Article15. Jeong KH, Lee TW, Ihm CG, et al. Real-Time Monitoring of the Effects of Telmisartan on Angiotensin II-Induced Mechanical Changes in Live Mesangial Cells Using Atomic Force Microscopy. Kidney Blood Press Res. 2012. 35:573–582.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular imaging of membrane proteins and microfilaments using atomic force microscopy
- Investigation of morphological changes of HPS membrane caused by cecropin B through scanning electron microscopy and atomic force microscopy
- Changes in surface roughness of bracket and wire after experimental sliding - preliminary study using an atomic force microscopy
- Nano-mechanical Phenotype as a Promising Biomarker to Evaluate Cancer Development, Progression, and Anti-cancer Drug Efficacy
- Effects of Mitomycin C on Scleral Collagen Fibrils According to Atomic Force Microscopy