Hanyang Med Rev.
2015 Aug;35(3):180-185. 10.7599/hmr.2015.35.3.180.
The Continuation of Erlotinib Treatment in Non-Small Cell Lung Cancer Patients Whose Brain Lesion Is the Only Site of Progression: Prospective Pilot Study
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. silk.ahn@samsung.com
- KMID: 2168364
- DOI: http://doi.org/10.7599/hmr.2015.35.3.180
Abstract
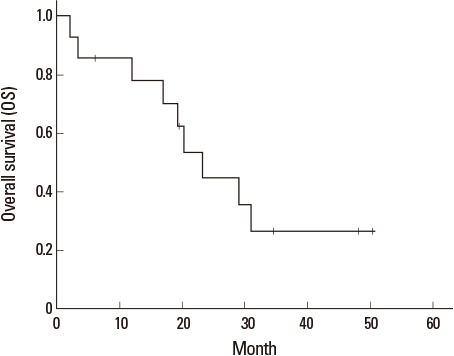
- There have been conflicting reports on the continuation of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) in patients with newly developed or progressive brain metastasis of non-small cell lung cancer (NSCLC). Patients with newly developed or progressive intracranial lesions, but who maintained well-controlled extracranial disease during erlotinib treatment, were enrolled in this study. The proposed therapy included stereotactic radiosurgery (SRS), whole brain radiotherapy (WBRT), and/or surgical resection for intracranial lesions. Erlotinib treatment was continued simultaneously unless extracranial disease progressed. The evaluation of both extra- and intra-cranial lesions was performed every 3 months. From October 2009 to June 2012, 14 patients were enrolled in this pilot study. For intracranial disease, 4 patients received SRS alone, 7 patients received both SRS and WBRT, 2 patients received SRS, WBRT and surgical resection, and 1 patient received no local therapy due to the presence of asymptomatic lesions. Of the patients with extracranial disease who were placed on continued erlotinib therapy, 6 patients (42.9%) showed partial response (PR), while 7 patients (50.0%) remained in stable disease (SD). The progression-free survival (PFS) of extracranial and intracranial disease was 11.1 (range 1.6-34.6) and 10.2 (range 1.5-34.6) months, respectively. In 5 cases, brain lesions relapsed before the progression of extracranial disease. Overall survival (OS) was 22.6 (range 2.1-50.4) months. For NSCLC patients with progression of only intracranial disease during erlotinib treatment, the continuation of erlotinib in combination with local therapy to brain metastases can be an effective treatment option.
MeSH Terms
Figure
Reference
-
1. Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004; 22:3852–3859.
Article2. Hotta K, Fujiwara Y, Matsuo K, Suzuki T, Kiura K, Tabata M, et al. Recent improvement in the survival of patients with advanced nonsmall cell lung cancer enrolled in phase III trials of first-line, systemic chemotherapy. Cancer. 2007; 109:939–948.
Article3. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012; 30:419–425.
Article4. Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, Morris RE, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96:17–32.
Article5. Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23:Suppl 7. vii56–vii64.
Article6. Al-Shamy G, Sawaya R. Management of brain metastases: the indispensable role of surgery. J Neurooncol. 2009; 92:275–282.
Article7. Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004; 363:1665–1672.
Article8. Scoccianti S, Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol. 2012; 102:168–179.
Article9. Mehta MP, Paleologos NA, Mikkelsen T, Robinson PD, Ammirati M, Andrews DW, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010; 96:71–83.
Article10. Cedres S, Prat A, Martinez P, Pallisa E, Sala G, Andreu J, et al. Clinical surrogate markers of survival in advanced non-small cell lung cancer (NSCLC) patients treated with second-third line erlotinib. Lung Cancer. 2009; 66:257–261.
Article11. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011; 12:735–742.
Article12. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13:239–246.13. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–132.
Article14. Reck M, van Zandwijk N, Gridelli C, Baliko Z, Rischin D, Allan S, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010; 5:1616–1622.
Article15. Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012; 70:399–405.
Article16. Deng Y, Feng W, Wu J, Chen Z, Tang Y, Zhang H, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol. 2014; 2:116–120.
Article17. Omuro AM, Kris MG, Miller VA, Franceschi E, Shah N, Milton DT, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005; 103:2344–2348.
Article18. Lee YJ, Choi HJ, Kim SK, Chang J, Moon JW, Park IK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer. 2010; 116:1336–1343.
Article19. Welsh JW, Komaki R, Amini A, Munsell MF, Unger W, Allen PK, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013; 31:895–902.
Article20. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011; 17:6298–6303.
Article21. Lin NU. Targeted therapies in brain metastases. Curr Treat Options Neurol. 2014; 16:276.
Article22. Lee JC, Jang SH, Lee KY, Kim YC. Treatment of Non-small Cell Lung Carcinoma after Failure of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. Cancer Res Treat. 2013; 45:79–85.
Article23. Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007; 13:5150–5155.
Article24. Cho BC, Im CK, Park MS, Kim SK, Chang J, Park JP, et al. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol. 2007; 25:2528–2533.
Article25. Becker A, Crombag L, Heideman DA, Thunnissen FB, van Wijk AW, Postmus PE, et al. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer. 2011; 47:2603–2606.
Article26. Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006; 24:4517–4520.
Article27. Kuiper JL, Smit EF. High-dose, pulsatile erlotinib in two NSCLC patients with leptomeningeal metastases--one with a remarkable thoracic response as well. Lung Cancer. 2013; 80:102–105.
Article28. Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001; 19:3267–3279.
Article29. Shukuya T, Takahashi T, Naito T, Kaira R, Ono A, Nakamura Y, et al. Continuous EGFR-TKI administration following radiotherapy for non-small cell lung cancer patients with isolated CNS failure. Lung Cancer. 2011; 74:457–461.
Article30. McHaffie DR, Chabot P, Dagnault A, Suh JH, Fortin MA, Chang E, et al. Safety and feasibility of motexafin gadolinium administration with whole brain radiation therapy and stereotactic radiosurgery boost in the treatment of </= 6 brain metastases: a multi-institutional phase II trial. J Neurooncol. 2011; 105:301–308.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ovarian Metastasis from Non-Small Cell Lung Cancer Responding to Erlotinib
- Recurrent Erlotinib-Induced Interstitial Lung Disease on Non-Small Cell Lung Cancer
- Erlotinib HCL (Tarceva(R)) Induced Radiation Recall Dermatitis
- Comparison of the therapeutic outcome between gefitinib and erlotinib in female patients with non-small-cell lung cancer
- Bowel Perforation after Erlotinib Treatment in a Patient with Non-Small Cell Lung Cancer