Hanyang Med Rev.
2013 May;33(2):110-117. 10.7599/hmr.2013.33.2.110.
Mass Spectrometry Analysis for Nitration of Proteins
- Affiliations
-
- 1Department of Molecular Biotechnology, Konkuk University, Seoul, Korea. kpkim@konkuk.ac.kr
- KMID: 2168241
- DOI: http://doi.org/10.7599/hmr.2013.33.2.110
Abstract
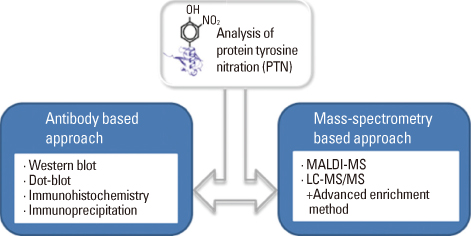
- Various proteomics and immunological methods including mass spectrometry combined with both liquid and 2-D PAGE, and immunodetection have been employed to identify and characterize nitrated proteins from pathological samples. Nitrosative modifications regulate cellular signal transduction and pathogenesis of inflammatory responses and neurodegenerative diseases. Nitric oxide generates reactive nitrosative species, such as peroxynitrite (ONOO-) that may be involved in a number of diseases. ONOO- can mediate protein tyrosine nitration which causes structural changes of affected proteins and leads to their inactivation. Protein tyrosine nitration is a biomarker of oxidative stress and also influences protein structure and function. Recent advances in mass spectrometry have made it possible to identify modified proteins and specific modified amino acid residues. This review focuses on the significance of protein tyrosine nitration and the progress achieved in analytical methods. Although mass spectrometry of nitrated peptides has become a powerful tool for the analysis of nitrated peptides, the low stoichiometry of protein tyrosine nitration clearly demands the use of affinity chromatography to enrich modified proteins (or peptides).
MeSH Terms
Figure
Cited by 1 articles
-
Do Reactive Oxygen Species Cause Aging?
Seong Eon Ryu
Hanyang Med Rev. 2013;33(2):75-76. doi: 10.7599/hmr.2013.33.2.75.
Reference
-
1. Reynolds MR, Berry RW, Binder LI. Site-specific nitration and oxidative dityrosine bridging of the tau protein by peroxynitrite: implications for Alzheimer's disease. Biochemistry. 2005; 44:1690–1700.
Article2. Yeo WS, Lee SJ, Lee JR, Kim KP. Nitrosative protein tyrosine modifications: biochemistry and functional significance. BMB Rep. 2008; 41:194–203.
Article3. Muntane J, la Mata MD. Nitric oxide and cancer. World J Hepatol. 2010; 2:337–344.
Article4. MacMillan-Crow LA, Cruthirds DL, Ahki KM, Sanders PW, Thompson JA. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol Med. 2001; 31:1603–1608.
Article5. Mann M, Hendrickson RC, Pandey A. Analysis of proteins and proteomes by mass spectrometry. Annu Rev Biochem. 2001; 70:437–473.
Article6. Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988; 60:2299–2301.
Article7. Abello N, Kerstjens HA, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J Proteome Res. 2009; 8:3222–3238.
Article8. Turko IV, Murad F. Mapping sites of tyrosine nitration by matrix-assisted laser desorption/ionization mass spectrometry. Methods Enzymol. 2005; 396:266–275.
Article9. Tedeschi G, Cappelletti G, Negri A, Pagliato L, Maggioni MG, Maci R, et al. Characterization of nitroproteome in neuron-like PC12 cells differentiated with nerve growth factor: identification of two nitration sites in alpha-tubulin. Proteomics. 2005; 5:2422–2432.
Article10. Sarver A, Scheffler NK, Shetlar MD, Gibson BW. Analysis of peptides and proteins containing nitrotyrosine by matrix-assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 2001; 12:439–448.
Article11. Sheeley SA, Rubakhin SS, Sweedler JV. The detection of nitrated tyrosine in neuropeptides: a MALDI matrix-dependent response. Anal Bioanal Chem. 2005; 382:22–27.
Article12. Petersson AS, Steen H, Kalume DE, Caidahl K, Roepstorff P. Investigation of tyrosine nitration in proteins by mass spectrometry. J Mass Spectrom. 2001; 36:616–625.
Article13. Salavej P, Spalteholz H, Arnhold J. Modification of amino acid residues in human serum albumin by myeloperoxidase. Free Radic Biol Med. 2006; 40:516–525.
Article14. Shin YS, Moon JH, Kim MS. Selective screening of tyrosine-nitrated peptides in tryptic mixtures by in-source photodissociation at 355 nm in matrix-assisted laser desorption ionization. Anal Chem. 2011; 83:1704–1708.
Article15. Butt YK, Lo SC. Detecting nitrated proteins by proteomic technologies. Methods Enzymol. 2008; 440:17–31.
Article16. Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004; 101:4003–4008.
Article17. Yoon SW, Kang S, Ryu SE, Poo H. Identification of tyrosine-nitrated proteins in HT22 hippocampal cells during glutamate-induced oxidative stress. Cell Prolif. 2010; 43:584–593.
Article18. Cappelletti G, Maggioni MG, Tedeschi G, Maci R. Protein tyrosine nitration is triggered by nerve growth factor during neuronal differentiation of PC12 cells. Exp Cell Res. 2003; 288:9–20.
Article19. Reed TT, Pierce WM Jr, Turner DM, Markesbery WR, Butterfield DA. Proteomic identification of nitrated brain proteins in early Alzheimer's disease inferior parietal lobule. J Cell Mol Med. 2009; 13:2019–2029.
Article20. Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer's disease brain. J Neurochem. 2003; 85:1394–1401.
Article21. Kanski J, Alterman MA, Schoneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic Biol Med. 2003; 35:1229–1239.
Article22. Salzano AM, D'Ambrosio C, Scaloni A. Mass spectrometric characterization of proteins modified by nitric oxide-derived species. Methods Enzymol. 2008; 440:3–15.
Article23. Fiore G, Di Cristo C, Monti G, Amoresano A, Columbano L, Pucci P, et al. Tubulin nitration in human gliomas. Neurosci Lett. 2006; 394:57–62.
Article24. Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, et al. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006; 22:76–87.
Article25. Zhan X, Desiderio DM. Nitroproteins Identified in Human Ex-smoker Bronchoalveolar Lavage Fluid. Aging Dis. 2011; 2:100–115.26. Zhan X, Desiderio DM. MALDI-induced Fragmentation of Leucine enkephalin, Nitro-Tyr Leucine Enkaphalin, and d(5)-Phe-Nitro-Tyr Leucine Enkephalin. Int J Mass Spectrom. 2009; 287:77–86.
Article27. Söderling AS, Hultman L, Delbro D, Højrup P, Caidahl K. Reduction of the nitro group during sample preparation may cause underestimation of the nitration level in 3-nitrotyrosine immunoblotting. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 851:277–286.
Article28. Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem. 2004; 279:27257–27262.
Article29. Borges CR, Kuhn DM, Watson JT. Mass mapping sites of nitration in tyrosine hydroxylase: random vs selective nitration of three tyrosine residues. Chem Res Toxicol. 2003; 16:536–540.
Article30. Ducrocq C, Dendane M, Laprevote O, Serani L, Das BC, Bouchemal-Chibani N, et al. Chemical modifications of the vasoconstrictor peptide angiotensin II by nitrogen oxides (NO, HNO2, HOONO)--evaluation by mass spectrometry. Eur J Biochem. 1998; 253:146–153.
Article31. Cook SL, Jackson GP. Characterization of tyrosine nitration and cysteine nitrosylation modifications by metastable atom-activation dissociation mass spectrometry. J Am Soc Mass Spectrom. 2011; 22:221–232.
Article32. Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, et al. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal Chem. 2000; 72:563–573.
Article33. Kelleher NL, Zubarev RA, Bush K, Furie B, Furie BC, McLafferty FW, et al. Localization of labile posttranslational modifications by electron capture dissociation: the case of gamma-carboxyglutamic acid. Anal Chem. 1999; 71:4250–4253.
Article34. Jones AW, Mikhailov VA, Iniesta J, Cooper HJ. Electron capture dissociation mass spectrometry of tyrosine nitrated peptides. J Am Soc Mass Spectrom. 2010; 21:268–277.
Article35. Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem. 2003; 278:33972–33977.
Article36. Zhan X, Desiderio DM. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal Biochem. 2006; 354:279–289.
Article37. Nikov G, Bhat V, Wishnok JS, Tannenbaum SR. Analysis of nitrated proteins by nitrotyrosine-specific affinity probes and mass spectrometry. Anal Biochem. 2003; 320:214–222.
Article38. Abello N, Barroso B, Kerstjens HA, Postma DS, Bischoff R. Chemical labeling and enrichment of nitrotyrosine-containing peptides. Talanta. 2010; 80:1503–1512.
Article39. Zhang Q, Qian WJ, Knyushko TV, Clauss TR, Purvine SO, Moore RJ, et al. A method for selective enrichment and analysis of nitrotyrosine-containing peptides in complex proteome samples. J Proteome Res. 2007; 6:2257–2268.
Article40. Prokai-Tatrai K, Guo J, Prokai L. Selective chemoprecipitation and subsequent release of tagged species for the analysis of nitropeptides by liquid chromatography-tandem mass spectrometry. Mol Cell Proteomics. 2011; 10:M110.002923.
Article41. Lee JR, Lee SJ, Kim TW, Kim JK, Park HS, Kim DE, et al. Chemical approach for specific enrichment and mass analysis of nitrated peptides. Anal Chem. 2009; 81:6620–6626.
Article42. Kim JK, Lee JR, Kang JW, Lee SJ, Shin GC, Yeo WS, et al. Selective enrichment and mass spectrometric identification of nitrated peptides using fluorinated carbon tags. Anal Chem. 2011; 83:157–163.
Article43. Crowley JR, Yarasheski K, Leeuwenburgh C, Turk J, Heinecke JW. Isotope dilution mass spectrometric quantification of 3-nitrotyrosine in proteins and tissues is facilitated by reduction to 3-aminotyrosine. Anal Biochem. 1998; 259:127–135.
Article44. Ghesquiere B, Colaert N, Helsens K, Dejager L, Vanhaute C, Verleysen K, et al. In vitro and in vivo protein-bound tyrosine nitration characterized by diagonal chromatography. Mol Cell Proteomics. 2009; 8:2642–2652.
Article45. Larsen TR, Bache N, Gramsbergen JB, Roepstorff P. Identification of nitrotyrosine containing peptides using combined fractional diagonal chromatography (COFRADIC) and off-line nano-LC-MALDI. J Am Soc Mass Spectrom. 2011; 22:989–996.
Article46. Sharov VS, Dremina ES, Galeva NA, Gerstenecker GS, Li X, Dobrowsky RT, et al. Fluorogenic Tagging of Peptide and Protein 3-Nitrotyrosine with 4-(Aminomethyl)-benzenesulfonic Acid for Quantitative Analysis of Protein Tyrosine Nitration. Chromatographia. 2010; 71:37–53.
Article47. Wisastra R, Poelstra K, Bischoff R, Maarsingh H, Haisma HJ, Dekker FJ. Antibody-free detection of protein tyrosine nitration in tissue sections. Chembiochem. 2011; 12:2016–2020.
Article48. Robinson RA, Evans AR. Enhanced sample multiplexing for nitrotyrosine-modified proteins using combined precursor isotopic labeling and isobaric tagging. Anal Chem. 2012; 84:4677–4686.
Article49. Zhang Y, Yang H, Poschl U. Analysis of nitrated proteins and tryptic peptides by HPLC-chip-MS/MS: site-specific quantification, nitration degree, and reactivity of tyrosine residues. Anal Bioanal Chem. 2011; 399:459–471.
Article50. Tsumoto H, Taguchi R, Kohda K. Efficient identification and quantification of peptides containing nitrotyrosine by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry after derivatization. Chem Pharm Bull (Tokyo). 2010; 58:488–494.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Novel Metabolic Proteins Released by Insulin Signaling of the Rat Hypothalmus Using Liquid Chromatography-Mass Spectrometry (LC-MS)
- Sporozoite proteome analysis of Cryptosporidium parvum by one-dimensional SDS-PAGE and liquid chromatography tandem mass spectrometry
- Charting the proteome of Cryptosporidium parvum sporozoites using sequence similarity-based BLAST searching
- Possible Roles of LAMMER Kinase Lkh1 in Fission Yeast by Comparative Proteome Analysis
- Comparison between Source-induced Dissociation and Collision-induced Dissociation of Ampicillin, Chloramphenicol, Ciprofloxacin, and Oxytetracycline via Mass Spectrometry