Immune Netw.
2014 Apr;14(2):89-99. 10.4110/in.2014.14.2.89.
Kinetics of IFN-gamma and IL-17 Production by CD4 and CD8 T Cells during Acute Graft-versus-Host Disease
- Affiliations
-
- 1Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 110-799, Korea. eycii@snu.ac.kr
- 2Biomedical Research Institute, Seoul National University Hospital, Seoul 110-799, Korea.
- KMID: 2168019
- DOI: http://doi.org/10.4110/in.2014.14.2.89
Abstract
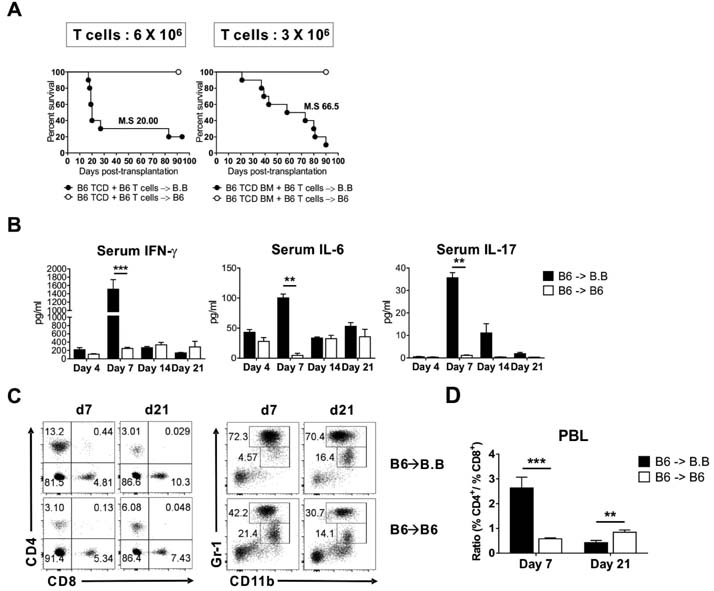
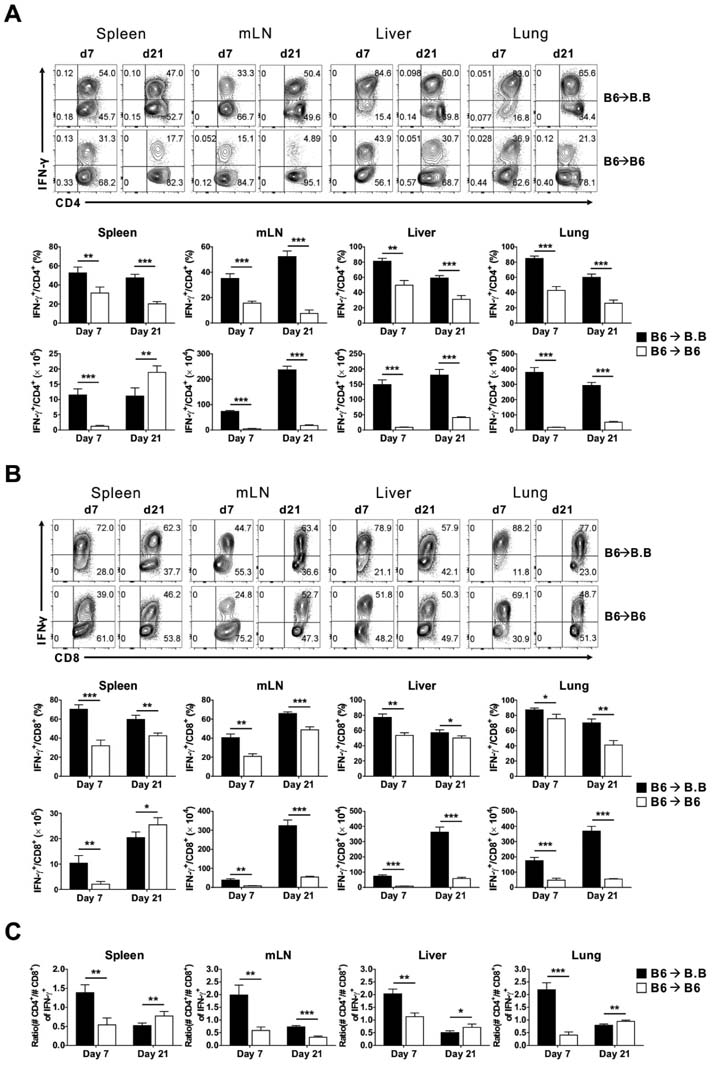
- Graft-versus-host disease (GVHD) is a fatal complication that occurs after allogeneic hematopoietic stem cell transplantation. To understand the dynamics of CD4 and CD8 T cell production of IFN-gamma and IL-17 during GVHD progression, we established a GVHD model by transplanting T cell-depleted bone marrow (TCD-BM) and purified T cells from B6 mice into irradiated BALB.B, creating an MHC-matched but minor histocompatibility (H) antigen-mismatched transplantation (B6 --> BALB.B GVHD). Transplantation-induced GVHD was confirmed by the presence of the appropriate compositional changes in the T cell compartments and innate immune cells in the blood and the systemic secretion of inflammatory cytokines. Using this B6 --> BALB.B GVHD model, we showed that the production of IFN-gamma and IL-17 by CD4 T cells preceded that by CD8 T cells in the spleen, mesenteric lymph node, liver, and lung in the BALB.B GVHD host, and Th1 differentiation predated Th17 differentiation in all organs during GVHD progression. Such changes in cytokine production were based on changes in cytokine gene expression by the T cells at different time points during GVHD development. These results demonstrate that both IFN-gamma and IL-17 are produced by CD4 and CD8 T cells but with different kinetics during GVHD progression.
MeSH Terms
Figure
Cited by 2 articles
-
TLR/MyD88-mediated Innate Immunity in Intestinal Graft-versus-Host Disease
Young-Kwan Lee, Myungsoo Kang, Eun Young Choi
Immune Netw. 2017;17(3):144-151. doi: 10.4110/in.2017.17.3.144.Skewed Dendritic Cell Differentiation of MyD88-Deficient Donor Bone Marrow Cells, Instead of Massive Expansion as Myeloid-Derived Suppressor Cells, Aggravates GVHD
Young-Kwan Lee, Ji-Min Ju, Woo-Jeong Shon, Sehwa Oh, Chang-Ki Min, Myung-Soo Kang, Dong-Mi Shin, Eun Young Choi
Immune Netw. 2018;18(6):. doi: 10.4110/in.2018.18.e44.
Reference
-
1. Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012; 12:443–458.
Article2. Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007; 7:340–352.
Article3. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation. Biol Blood Marrow Transplant. 2005; 11:945–956.
Article4. Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009; 114:4327–4336.
Article5. Sun Y, Tawara I, Toubai T, Reddy P. Pathophysiology of acute graft-versus-host disease: recent advances. Transl Res. 2007; 150:197–214.
Article6. Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999; 5:347–356.
Article7. Iclozan C, Yu Y, Liu C, Liang Y, Yi T, Anasetti C, Yu XZ. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010; 16:170–178.
Article8. Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu DT, Chen L, Iwakura Y, Kandeel F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009; 114:3101–3112.
Article9. Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998; 102:2126–2135.
Article10. Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008; 8:337–348.
Article11. Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in alloimmunity: a novel IL-17 antagonist promotes heart graft survival. Transplant Proc. 1999; 31:93.
Article12. Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002; 197:322–332.
Article13. Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012; 18:S56–S61.
Article14. Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009; 113:1365–1374.
Article15. Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009; 113:945–952.
Article16. Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, LeBon T, Kandeel F, Forman S, Zeng D. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008; 112:2101–2110.
Article17. Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, Anders RA, Trimble CL, Adler AJ, Lin TY, Pardoll DM, Huang CT, Drake CG. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol. 2009; 183:7161–7168.
Article18. Lai HY, Chou TY, Tzeng CH, Lee OK. Cytokine profiles in various graft-versus-host disease target organs following hematopoietic stem cell transplantation. Cell Transplant. 2012; 21:2033–2045.
Article19. Choi EY, Christianson GJ, Yoshimura Y, Jung N, Sproule TJ, Malarkannan S, Joyce S, Roopenian DC. Real-time T-cell profiling identifies H60 as a major minor histocompatibility antigen in murine graft-versus-host disease. Blood. 2002; 100:4259–4265.
Article20. Song MG, Kang B, Jeon JY, Chang J, Lee S, Min CK, Youn H, Choi EY. In Vivo imaging of differences in early donor cell proliferation in graft-versus-host disease hosts with different pre-conditioning doses. Mol Cells. 2012; 33:79–86.
Article21. Choi JH, Yoon H, Min CK, Choi EY. Effects of pre-conditioning dose on the immune kinetics and cytokine production in the leukocytes infiltrating GVHD tissues after MHC-matched transplantation. Immune Netw. 2011; 11:68–78.
Article22. Toubai T, Tanaka J, Paczesny S, Shono Y, Reddy P, Imamura M. Role of cytokines in the pathophysiology of acute graft-versus-host disease (GVHD): are erum/plasma cytokines potential biomarkers for diagnosis of acute GVHD following allogeneic hematopoietic cell transplantation (Allo-HCT)? Curr Stem Cell Res Ther. 2012; 7:229–239.
Article23. Murai M, Yoneyama H, Ezaki T, Suematsu M, Terashima Y, Harada A, Hamada H, Asakura H, Ishikawa H, Matsushima K. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol. 2003; 4:154–160.
Article24. Panoskaltsis-Mortari A, Price A, Hermanson JR, Taras E, Lees C, Serody JS, Blazar BR. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004; 103:3590–3598.
Article25. Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005; 106:1113–1122.
Article26. Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, Lineburg KE, Cheong M, Robb RJ, Markey KA, Varelias A, Malissen B, Hämmerling GJ, Clouston AD, Engwerda CR, Bhat P, MacDonald KP, Hill GR. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med. 2011; 18:135–142.
Article27. Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001; 29:259–277.
Article28. Visentainer JE, Lieber SR, Persoli LB, Vigorito AC, Aranha FJ, de Brito Eid KA, Oliveira GB, Miranda EC, de Souza CA. Serum cytokine levels and acute graft-versus-host disease after HLA-identical hematopoietic stem cell transplantation. Exp Hematol. 2003; 31:1044–1050.
Article29. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997; 90:3204–3213.
Article30. Nishimori H, Maeda Y, Teshima T, Sugiyama H, Kobayashi K, Yamasuji Y, Kadohisa S, Uryu H, Takeuchi K, Tanaka T, Yoshino T, Iwakura Y, Tanimoto M. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by down-regulating Th1 and Th17. Blood. 2012; 119:285–295.
Article31. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005; 6:1133–1141.
Article32. O'Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008; 181:3750–3754.33. Zhao F, Zhang Y, Wang H, Jin M, He S, Shi Y, Guo Y, Zhang Y. Blockade of osteopontin reduces alloreactive CD8+ T cell-mediated graft-versus-host disease. Blood. 2011; 117:1723–1733.
Article34. Broady R, Yu J, Chow V, Tantiworawit A, Kang C, Berg K, Martinka M, Ghoreishi M, Dutz J, Levings MK. Cutaneous GVHD is associated with the expansion of tissue-localized Th1 and not Th17 cells. Blood. 2010; 116:5748–5751.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Production of interleukin 4 and interferon gamma in CD8+ T cells from patients with intrinsic and extrinsic asthma
- Canges of Peripheral IFN-r-Producing CD4(+) and CD4(-) Cell Frequencies in Kawasaki Disease
- The production of interferon gamma by peripheral T lymphocytes in patients with allergic asthma upon stimulation with Der p2 antigen
- Maintenance of CD8+T-cell anergy by CD4+CD25+ regulatory T cells in chronic graft-versus-host disease
- Anti-CD137 mAb Deletes Both Donor CD4+ and CD8+ T Cells in Acute Graft-versus-host Disease