Cancer Res Treat.
2014 Apr;46(2):172-177.
Modified MVAC as a Second-Line Treatment for Patients with Metastatic Urothelial Carcinoma after Failure of Gemcitabine and Cisplatin Treatment
- Affiliations
-
- 1Korea University College of Medicine, Seoul, Korea.
- 2Department of Urology, Korea University College of Medicine, Seoul, Korea.
- 3Division of Oncology/Hematology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. khpark@korea.ac.kr
- 4Department of Radiation Oncology, Korea University College of Medicine, Seoul, Korea.
- 5Department of Radiology, Korea University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
There is no established standard second-line chemotherapy for patients with advanced or metastatic urothelial carcinoma (UC) who failed gemcitabine and cisplatin (GC) chemotherapy. This study was conducted in order to investigate the efficacy and toxicity of modified methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) in patients with metastatic UC previously treated with GC.
MATERIALS AND METHODS
We retrospectively analyzed 28 patients who received modified MVAC between November 2004 and November 2012. All patients failed prior, first-line GC chemotherapy.
RESULTS
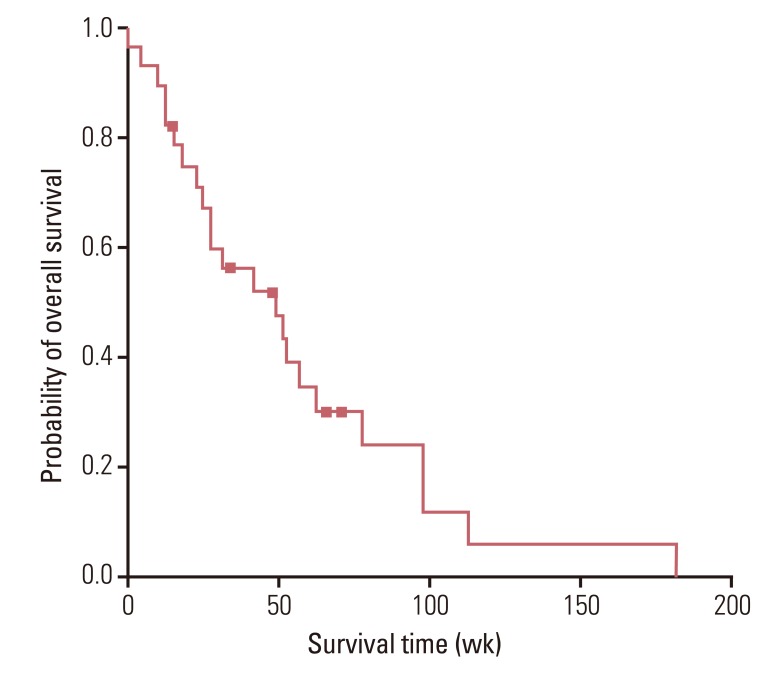
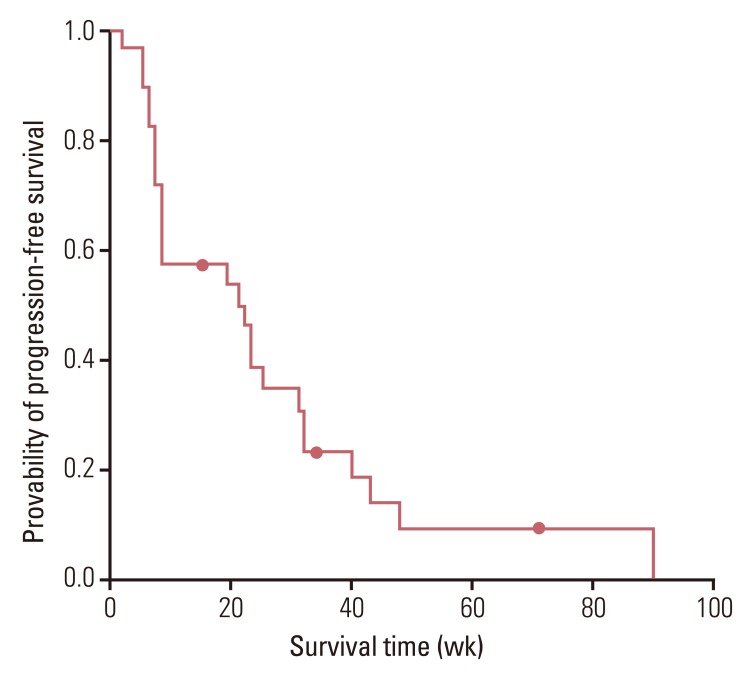
The median age of patients was 64.0 years (range, 33.0 to 77.0 years), and 23 (82.1%) patients had an Eastern Cooperative Oncology Group performance status of 0 or 1. The overall response rate and the disease control rate were 36.0% and 64.0%, respectively. After a median follow-up period of 38 weeks (range, 5 to 182 weeks), median progression free survival was 21.0 weeks (95% confidence interval [CI], 6.3 to 35.7 weeks) and median overall survival was 49.0 weeks (95% CI, 18.8 to 79.3 weeks). Grade 3 or 4 hematological toxicities included neutropenia (n=21, 75.0%) and anemia (n=9, 32.1%). Grade 3 or 4 non-hematological toxicities did not occur and there was no treatment-related death.
CONCLUSION
Modified MVAC appears to be a safe and active chemotherapy regimen in patients with stable physical status and adequate renal function after GC treatment.
MeSH Terms
Figure
Reference
-
1. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011; 43:1–11. PMID: 21509157.
Article2. Raghavan D. Advanced bladder and urothelial cancers. Eur J Cancer. 2000; 36(Suppl 2):1–6. PMID: 10908841.
Article3. Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009; 55:815–825. PMID: 19157687.
Article4. Bellmunt J, Orsola A, Wiegel T, Guix M, De Santis M, Kataja V, et al. Bladder cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22(Suppl 6):vi45–vi49. PMID: 21908503.
Article5. Bamias A, Tiliakos I, Karali MD, Dimopoulos MA. Systemic chemotherapy in inoperable or metastatic bladder cancer. Ann Oncol. 2006; 17:553–561. PMID: 16303860.
Article6. Dreicer R, Manola J, Schneider DJ, Schwerkoske JF, George CS, Roth BJ, et al. Phase II trial of gemcitabine and docetaxel in patients with advanced carcinoma of the urothelium: a trial of the Eastern Cooperative Oncology Group. Cancer. 2003; 97:2743–2747. PMID: 12767086.7. Witte RS, Elson P, Bono B, Knop R, Richardson RR, Dreicer R, et al. Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol. 1997; 15:589–593. PMID: 9053481.
Article8. Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol. 2002; 20:937–940. PMID: 11844814.
Article9. Dreicer R, Li S, Manola J, Haas NB, Roth BJ, Wilding G, et al. Phase 2 trial of epothilone B analog BMS-247550 (ixabepilone) in advanced carcinoma of the urothelium (E3800): a trial of the Eastern Cooperative Oncology Group. Cancer. 2007; 110:759–763. PMID: 17594721.10. von der Maase H. Gemcitabine in transitional cell carcinoma of the urothelium. Expert Rev Anticancer Ther. 2003; 3:11–19. PMID: 12597345.
Article11. Sweeney CJ, Roth BJ, Kabbinavar FF, Vaughn DJ, Arning M, Curiel RE, et al. Phase II study of pemetrexed for second-line treatment of transitional cell cancer of the urothelium. J Clin Oncol. 2006; 24:3451–3457. PMID: 16849761.
Article12. Vaughn DJ, Srinivas S, Stadler WM, Pili R, Petrylak D, Sternberg CN, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 study. Cancer. 2009; 115:4110–4117. PMID: 19536904.13. Sternberg CN, Yagoda A, Scher HI, Watson RC, Geller N, Herr HW, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium: efficacy and patterns of response and relapse. Cancer. 1989; 64:2448–2458. PMID: 2819654.
Article14. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000; 18:3068–3077. PMID: 11001674.
Article15. Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006; 42:50–54. PMID: 16330205.
Article16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.
Article17. Kaufman D, Raghavan D, Carducci M, Levine EG, Murphy B, Aisner J, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol. 2000; 18:1921–1927. PMID: 10784633.
Article18. Tannock I, Gospodarowicz M, Connolly J, Jewett M. M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) chemotherapy for transitional cell carcinoma: the Princess Margaret Hospital experience. J Urol. 1989; 142(2 Pt 1):289–292. PMID: 2746745.
Article19. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005; 23:4602–4608. PMID: 16034041.
Article20. de Wit R. European Organization for Research and Treatment. Overview of bladder cancer trials in the European Organization for Research and Treatment. Cancer. 2003; 97(8 Suppl):2120–2126. PMID: 12673705.
Article21. Suyama T, Ueda T, Fukasawa S, Imamura Y, Nakamura K, Miyasaka K, et al. Combination of gemcitabine and paclitaxel as second-line chemotherapy for advanced urothelial carcinoma. Jpn J Clin Oncol. 2009; 39:244–250. PMID: 19211575.
Article22. Han KS, Joung JY, Kim TS, Jeong IG, Seo HK, Chung J, et al. Methotrexate, vinblastine, doxorubicin and cisplatin combination regimen as salvage chemotherapy for patients with advanced or metastatic transitional cell carcinoma after failure of gemcitabine and cisplatin chemotherapy. Br J Cancer. 2008; 98:86–90. PMID: 18087289.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy in Advanced Urothelial Carcinoma
- Efficacy and Toxicity of Gemcitabine Plus Cisplatin Chemotherapy in Advanced Urothelial Cancer
- Hematologic Toxicity of Gemcitabine and Cisplatin Combination Therapy in Advanced Urothelial Cancer
- Efficacy and Toxicity of Gemcitabine Based Chemotherapy for Advanced Urothelial Cancer
- Treatment of MIBC - Neoadjuvant Chemotherapy: New Standard of Care