Cancer Res Treat.
2014 Jan;46(1):81-92.
Synergistic Effect of COX-2 Inhibitor on Paclitaxel-Induced Apoptosis in the Human Ovarian Cancer Cell Line OVCAR-3
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Institute of Women's Life Medical Science, Yonsei University College of Medicine, Seoul, Korea. ytkchoi@yuhs.ac
Abstract
- PURPOSE
Celecoxib, a highly selective cyclooxygenase-2 inhibitor, regulates apoptosis of several types of human cancer cells. The purpose of this study was to investigate whether celecoxib in combination with paclitaxel modulates apoptosis of ovarian cancer cells, and to identify the signal pathway by which celecoxib mediates apoptosis.
MATERIALS AND METHODS
OVCAR-3 cells were exposed to paclitaxel (20 microM) in the absence or presence of celecoxib (10 microM). Cell viability was evaluated using a Cell Counting Kit-8 assay. Apoptosis was evaluated using Annexin-V/7-aminoactinomycin D staining and a cellular DNA fragmentation enzyme-linked immunosorbent assay. Caspase-3, -9, and cleavage of poly ADP-ribose polymerase (PARP) were determined by western blotting. Expression of nuclear factor-kappaB (NF-kappaB) and vascular endothelial growth factor (VEGF) and Akt activation were assessed using reverse transcriptase-polymerase chain reaction and western blotting.
RESULTS
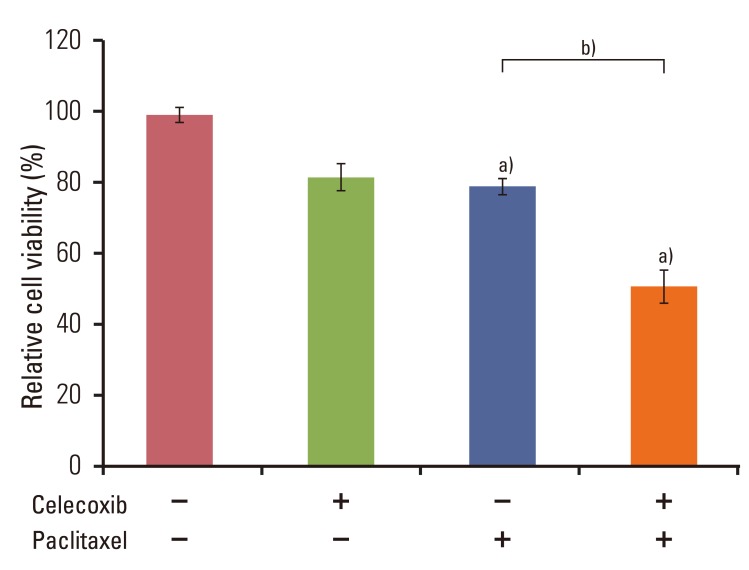
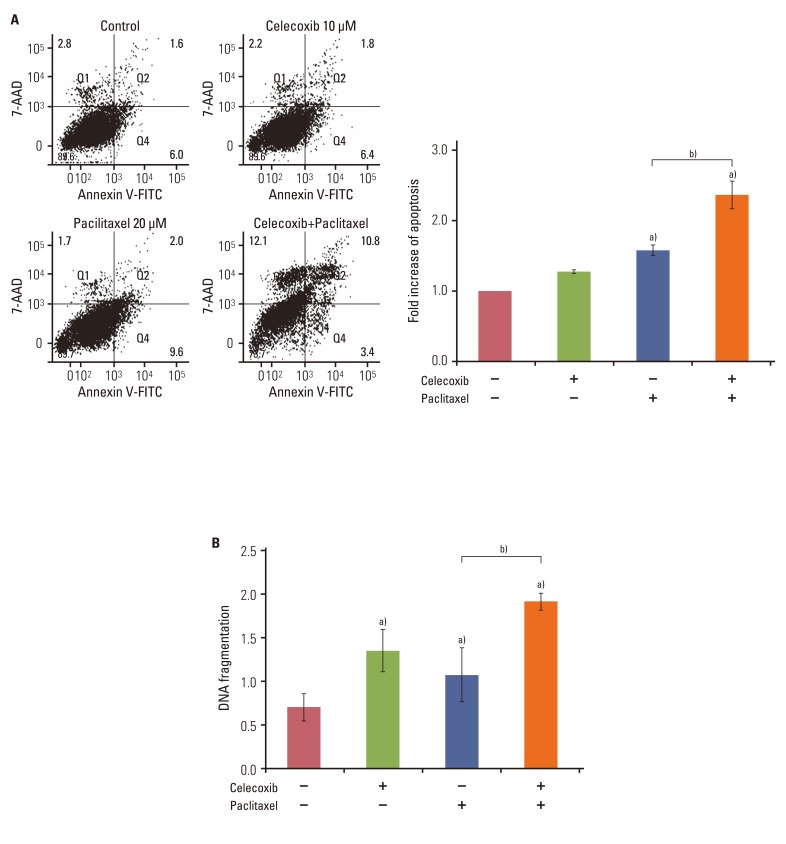
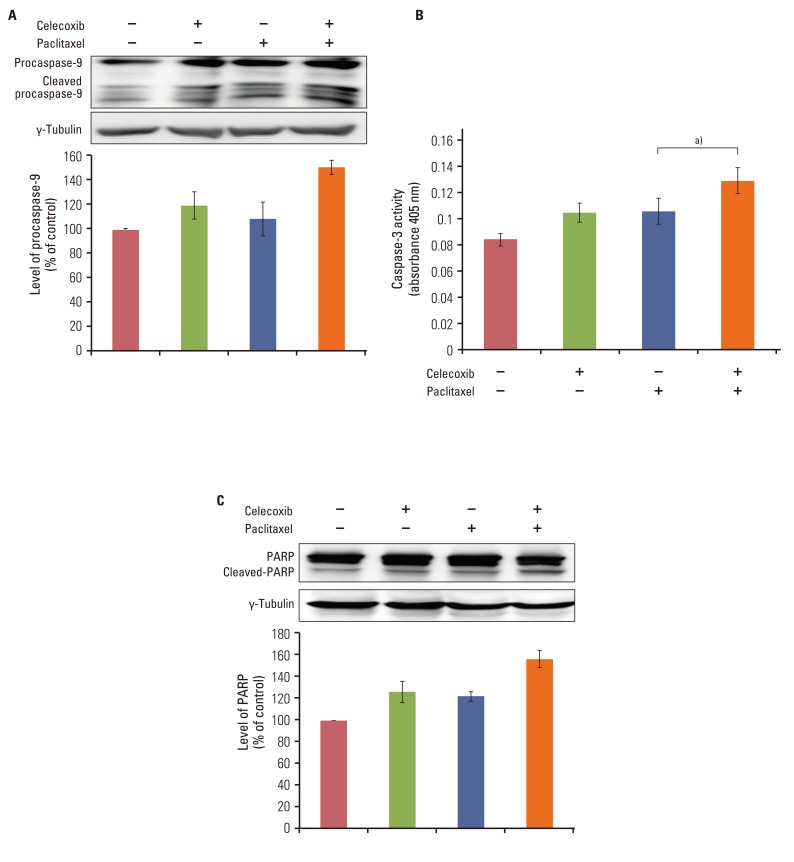
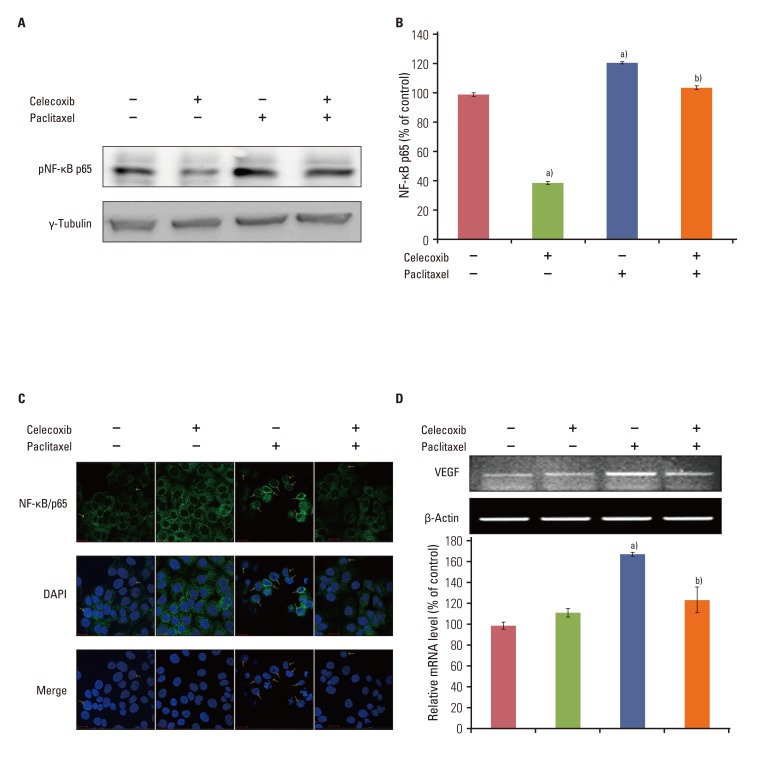
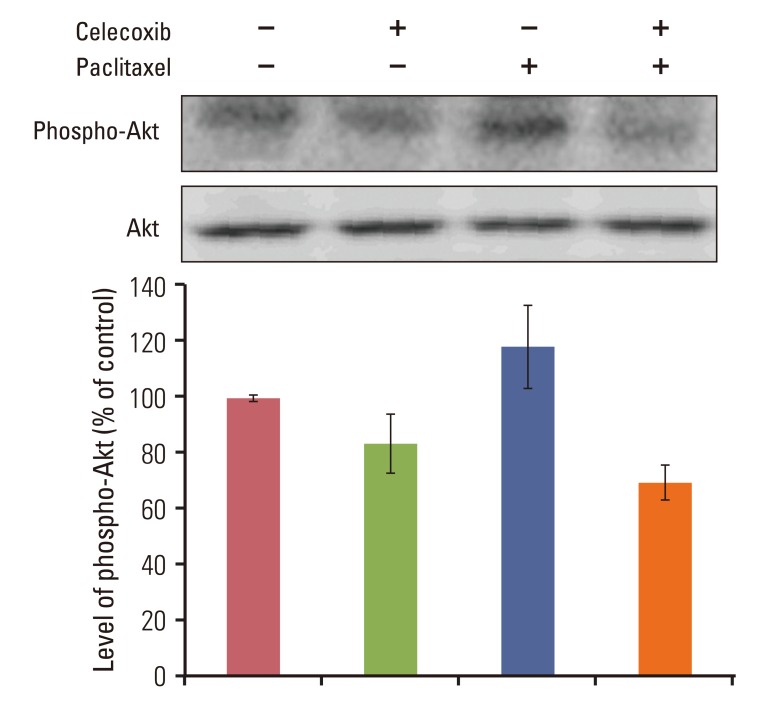
Celecoxib enhanced paclitaxel-induced growth inhibition of OVCAR-3 cells. Celecoxib significantly increased paclitaxel-induced apoptosis of OVCAR-3 cells. Pretreatment with celecoxib also increased activation of caspase-9, -3 and cleaved PARP following paclitaxel-treatment. Exposure of OVCAR-3 cells to celecoxib in combination with paclitaxel resulted in downregulation of NF-kappaB activation and VEGF expression. Furthermore, combining celecoxib and paclitaxel inhibited phosphorylation of Akt.
CONCLUSION
OVCAR-3 cells were sensitized to paclitaxel-induced apoptosis by celecoxib through downregulation of NF-kappaB and Akt activation, suggesting that celecoxib may work synergistically with paclitaxel to inhibit different targets and ultimately produce anticancer effects. Combining celecoxib with paclitaxel may prove beneficial in the clinical treatment of ovarian cancer.
Keyword
MeSH Terms
-
Adenosine Diphosphate Ribose
Apoptosis*
Blotting, Western
Caspase 3
Caspase 9
Cell Count
Cell Line*
Cell Survival
Cyclooxygenase 2
DNA Fragmentation
Down-Regulation
Enzyme-Linked Immunosorbent Assay
Humans*
NF-kappa B
Ovarian Neoplasms*
Paclitaxel
Phosphorylation
Signal Transduction
Vascular Endothelial Growth Factor A
Celecoxib
Adenosine Diphosphate Ribose
Caspase 3
Caspase 9
Cyclooxygenase 2
NF-kappa B
Paclitaxel
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. NIH consensus conference. Ovarian cancer. Screening, treatment, and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA. 1995; 273:491–497. PMID: 7837369.2. Kohn EC, Sarosy G, Bicher A, Link C, Christian M, Steinberg SM, et al. Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. J Natl Cancer Inst. 1994; 86:18–24. PMID: 7505830.
Article3. Kim SH, Juhnn YS, Song YS. Akt involvement in paclitaxel chemoresistance of human ovarian cancer cells. Ann N Y Acad Sci. 2007; 1095:82–89. PMID: 17404021.
Article4. Kutuk O, Letai A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008; 68:7985–7994. PMID: 18829556.
Article5. Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006; 66:3859–3868. PMID: 16585214.
Article6. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999; 18:7908–7916. PMID: 10630643.
Article7. Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001; 15:2742–2744. PMID: 11606477.8. Vandoros GP, Konstantinopoulos PA, Sotiropoulou-Bonikou G, Kominea A, Papachristou GI, Karamouzis MV, et al. PPAR-gamma is expressed and NF-κB pathway is activated and correlates positively with COX-2 expression in stromal myofibroblasts surrounding colon adenocarcinomas. J Cancer Res Clin Oncol. 2006; 132:76–84. PMID: 16215757.9. Ferrandina G, Lauriola L, Distefano MG, Zannoni GF, Gessi M, Legge F, et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol. 2002; 20:973–981. PMID: 11844819.
Article10. Chen C, Shen HL, Yang J, Chen QY, Xu WL. Preventing chemoresistance of human breast cancer cell line, MCF-7 with celecoxib. J Cancer Res Clin Oncol. 2011; 137:9–17. PMID: 20229271.
Article11. Lampiasi N, Azzolina A, Umezawa K, Montalto G, McCubrey JA, Cervello M. The novel NF-kappaB inhibitor DHMEQ synergizes with celecoxib to exert antitumor effects on human liver cancer cells by a ROS-dependent mechanism. Cancer Lett. 2012; 322:35–44. PMID: 22343223.12. Hermine O, Dubart A, Porteux F, Mayeux P, Titeux M, Dumenil D, et al. Inhibition of the erythropoietin-induced erythroid differentiation by granulocyte-macrophage colony-stimulating factor in the human UT-7 cell line is not due to a negative regulation of the erythropoietin receptor. Blood. 1996; 87:1746–1753. PMID: 8634420.
Article13. Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999; 5:119–127. PMID: 9918209.14. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002; 2:489–501. PMID: 12094235.15. Rodriguez-Burford C, Barnes MN, Oelschlager DK, Myers RB, Talley LI, Partridge EE, et al. Effects of nonsteroidal anti-inflammatory agents (NSAIDs) on ovarian carcinoma cell lines: preclinical evaluation of NSAIDs as chemopreventive agents. Clin Cancer Res. 2002; 8:202–209. PMID: 11801560.16. Bhatt RS, Merchan J, Parker R, Wu HK, Zhang L, Seery V, et al. A phase 2 pilot trial of low-dose, continuous infusion, or "metronomic" paclitaxel and oral celecoxib in patients with metastatic melanoma. Cancer. 2010; 116:1751–1756. PMID: 20120033.
Article17. Mutter R, Lu B, Carbone DP, Csiki I, Moretti L, Johnson DH, et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res. 2009; 15:2158–2165. PMID: 19276291.
Article18. Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004; 279:23477–23485. PMID: 15026414.19. Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, et al. Inhibition of inhibitor of nuclear factor-kappaB phosphorylation increases the efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Clin Cancer Res. 2004; 10:7645–7654. PMID: 15569997.20. Huang Y, Fan W. IkappaB kinase activation is involved in regulation of paclitaxel-induced apoptosis in human tumor cell lines. Mol Pharmacol. 2002; 61:105–113. PMID: 11752211.21. Yang G, Xiao X, Rosen DG, Cheng X, Wu X, Chang B, et al. The biphasic role of NF-kappaB in progression and chemoresistance of ovarian cancer. Clin Cancer Res. 2011; 17:2181–2194. PMID: 21339307.22. Nguyen DM, Chen GA, Reddy R, Tsai W, Schrump WD, Cole G Jr, et al. Potentiation of paclitaxel cytotoxicity in lung and esophageal cancer cells by pharmacologic inhibition of the phosphoinositide 3-kinase/protein kinase B (Akt)-mediated signaling pathway. J Thorac Cardiovasc Surg. 2004; 127:365–375. PMID: 14762343.
Article23. Kucab JE, Lee C, Chen CS, Zhu J, Gilks CB, Cheang M, et al. Celecoxib analogues disrupt Akt signaling, which is commonly activated in primary breast tumours. Breast Cancer Res. 2005; 7:R796–R807. PMID: 16168126.
Article24. Zhi YH, Liu RS, Song MM, Tian Y, Long J, Tu W, et al. Cyclooxygenase-2 promotes angiogenesis by increasing vascular endothelial growth factor and predicts prognosis in gallbladder carcinoma. World J Gastroenterol. 2005; 11:3724–3728. PMID: 15968728.
Article25. Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004; 51:239–247. PMID: 15254653.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the Specific COX-2 Inhibitor, Celecoxib, on Paclitaxel-Induced Apoptosis in SK-OV-3 Epithelial Ovarian Cancer Cell Line
- Flow Cytometric Detection of Apoptosis and p53 Protein in OVCAR-3 and SKOV-3 Ovarian Cancer Cell Lines
- Selective Protection of Normal Proliferationg Cells Against Anti-Neoplastic Chemotherapy by Stausporine Pretreatment
- Study on Measure of Chemosensitivities to Topotecan, Cisplatin and Taxol Theraphy in Ovarian Cancer Cell Lines: Relationship with p53 and bcl-2 Expression and Apoptosis
- Association of Type IV Collagenase Activation Potential With Metastatic Progression in Human Ovarian Carcinoma Cell Lines