Cancer Res Treat.
2013 Dec;45(4):251-262.
Targeting Arginine-Dependent Cancers with Arginine-Degrading Enzymes: Opportunities and Challenges
- Affiliations
-
- 1Center for Molecular Oncology, Barts Cancer Institute - a Cancer Research UK Centre of Excellence, Queen Mary University of London, Barts and The London School of Medicine and Dentistry, London, UK. p.w.szlosarek@qmul.ac.uk
- 2St Bartholomew's Hospital, London, UK.
- 3Pathology Group, Institute of Cell and Molecular Sciences, Queen Mary University of London, Barts and The London School of Medicine and Dentistry, London, UK.
Abstract
- Arginine deprivation is a novel antimetabolite strategy for the treatment of arginine-dependent cancers that exploits differential expression and regulation of key urea cycle enzymes. Several studies have focused on inactivation of argininosuccinate synthetase 1 (ASS1) in a range of malignancies, including melanoma, hepatocellular carcinoma (HCC), mesothelial and urological cancers, sarcomas, and lymphomas. Epigenetic silencing has been identified as a key mechanism for loss of the tumor suppressor role of ASS1 leading to tumoral dependence on exogenous arginine. More recently, dysregulation of argininosuccinate lyase has been documented in a subset of arginine auxotrophic glioblastoma multiforme, HCC and in fumarate hydratase-mutant renal cancers. Clinical trials of several arginine depletors are ongoing, including pegylated arginine deiminase (ADI-PEG20, Polaris Group) and bioengineered forms of human arginase. ADI-PEG20 is furthest along the path of clinical development from combinatorial phase 1 to phase 3 trials and is described in more detail. The challenge will be to identify tumors sensitive to drugs such as ADI-PEG20 and integrate these agents into multimodality drug regimens using imaging and tissue/fluid-based biomarkers as predictors of response. Lastly, resistance pathways to arginine deprivation require further study to optimize arginine-targeted therapies in the oncology clinic.
Keyword
MeSH Terms
Figure
Reference
-
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674. PMID: 21376230.
Article2. Schiffman JD. No child left behind in SDHB testing for paragangliomas and pheochromocytomas. J Clin Oncol. 2011; 29:4070–4072. PMID: 21969491.
Article3. Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011; 10:671–684. PMID: 21878982.
Article4. Broome JD. Evidence that the L-asparaginase activity of Guinea pig serum is responsible for its antilymphoma effects. Nature. 1961; 191:1114–1115.
Article5. Richards NG, Kilberg MS. Asparagine synthetase chemotherapy. Annu Rev Biochem. 2006; 75:629–654. PMID: 16756505.
Article6. Jaffe N, Traggis D, Das L, Frauenberger G, Hann HW, Kim BS, et al. Favorable remission induction rate with twice weekly doses of L-asparaginase. Cancer Res. 1973; 33:1–4. PMID: 4565907.7. Douer D. Is asparaginase a critical component in the treatment of acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2008; 21:647–658. PMID: 19041604.
Article8. Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia. 2013; 27:553–559. PMID: 23099335.
Article9. Offman MN, Krol M, Patel N, Krishnan S, Liu J, Saha V, et al. Rational engineering of L-asparaginase reveals importance of dual activity for cancer cell toxicity. Blood. 2011; 117:1614–1621. PMID: 21106986.
Article10. Wheatley DN. Controlling cancer by restricting arginine availability: arginine-catabolizing enzymes as anticancer agents. Anticancer Drugs. 2004; 15:825–833. PMID: 15457122.11. Feun L, Savaraj N. Pegylated arginine deiminase: a novel anticancer enzyme agent. Expert Opin Investig Drugs. 2006; 15:815–822.
Article12. Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003; 270:1887–1899. PMID: 12709047.
Article13. Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, et al. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004; 100:826–833. PMID: 14770441.14. Szlosarek PW, Grimshaw MJ, Wilbanks GD, Hagemann T, Wilson JL, Burke F, et al. Aberrant regulation of argininosuccinate synthetase by TNF-alpha in human epithelial ovarian cancer. Int J Cancer. 2007; 121:6–11. PMID: 17354225.15. Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010; 126:2762–2772. PMID: 20104527.
Article16. Delage B, Luong P, Maharaj L, O'Riain C, Syed N, Crook T, et al. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012; 3:e342. PMID: 22764101.
Article17. Tsai WB, Aiba I, Lee SY, Feun L, Savaraj N, Kuo MT. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther. 2009; 8:3223–3233. PMID: 19934275.18. Kobayashi E, Masuda M, Nakayama R, Ichikawa H, Satow R, Shitashige M, et al. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther. 2010; 9:535–544. PMID: 20159990.
Article19. Huang HY, Wu WR, Wang YH, Wang JW, Fang FM, Tsai JW, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013; 19:2861–2872. PMID: 23549872.
Article20. Allen M, Luong P, Hudson C, Leyton J, Delage B, Ghazaly E, et al. Prognostic and therapeutic impact of argininosuccinate synthetase-1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2013; 11. 27. [Epub]. http://dx.doi.org/10.1158/0008-5472.CAN-13-1702 .
Article21. Osunkoya BO, Adler WH, Smith RT. Effect of arginine deficiency on synthesis of DNA and immunoglobulin receptor of Burkitt lymphoma cells. Nature. 1970; 227:398–399. PMID: 4193643.
Article22. Yamauchi K, Komatsu T, Kulkarni AD, Ohmori Y, Minami H, Ushiyama Y, et al. Glutamine and arginine affect Caco-2 cell proliferation by promotion of nucleotide synthesis. Nutrition. 2002; 18:329–333. PMID: 11934546.
Article23. Long Y, Tsai WB, Wangpaichitr M, Tsukamoto T, Savaraj N, Feun LG, et al. Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol Cancer Ther. 2013; 12:2581–2590. PMID: 23979920.
Article24. Braas D, Ahler E, Tam B, Nathanson D, Riedinger M, Benz MR, et al. Metabolomics strategy reveals subpopulation of liposarcomas sensitive to gemcitabine treatment. Cancer Discov. 2012; 2:1109–1117. PMID: 23230188.
Article25. Van Tine BA, Bean GR, Boone P, Tanas M, Schulze MB, Chen DY, et al. Using pegylated arginine deiminase (ADI-PEG20) for the treatment of sarcomas that lack argininosuccinate synthesase 1 expression. J Clin Oncol. 2013; 31(S):10526.
Article26. Lan J, Tai HC, Lee SW, Chen TJ, Huang HY, Li CF. Deficiency in expression and epigenetic DNA methylation of ASS1 gene in nasopharyngeal carcinoma: negative prognostic impact and therapeutic relevance. Tumour Biol. 2013; 7. 30. [Epub]. http://dx.doi.org/10.1007/s13277-013-1020-8 .
Article27. Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011; 476:346–350. PMID: 21760589.
Article28. Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. 2011; 17:1619–1626. PMID: 22081021.
Article29. Huang HL, Hsu HP, Shieh SC, Chang YS, Chen WC, Cho CY, et al. Attenuation of argininosuccinate lyase inhibits cancer growth via cyclin A2 and nitric oxide. Mol Cancer Ther. 2013; 12:2505–2516. PMID: 23979921.
Article30. Syed N, Langer J, Janczar K, Singh P, Lo Nigro C, Lattanzio L, et al. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 2013; 4:e458. PMID: 23328665.
Article31. Zheng L, MacKenzie ED, Karim SA, Hedley A, Blyth K, Kalna G, et al. Reversed argininosuccinate lyase activity in fumarate hydratase-deficient cancer cells. Cancer Metab. 2013; 1:12. PMID: 24280230.
Article32. Adam J, Yang M, Bauerschmidt C, Kitagawa M, O'Flaherty L, Maheswaran P, et al. A role for cytosolic fumarate hydratase in urea cycle metabolism and renal neoplasia. Cell Rep. 2013; 3:1440–1448. PMID: 23643539.
Article33. Feun LG, Marini A, Walker G, Elgart G, Moffat F, Rodgers SE, et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer. 2012; 106:1481–1485. PMID: 22472884.
Article34. Kelly MP, Jungbluth AA, Wu BW, Bomalaski J, Old LJ, Ritter G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br J Cancer. 2012; 106:324–332. PMID: 22134507.
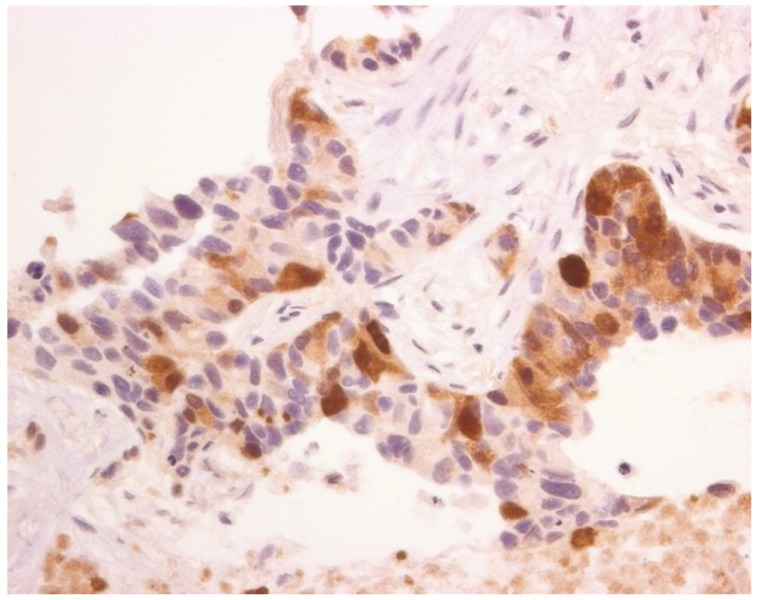
Article35. Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T, et al. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006; 12:7126–7131. PMID: 17145837.
Article36. Gilroy E. The influence of arginine upon the growth rate of a transplantable tumour in the mouse. Biochem J. 1930; 24:589–595. PMID: 16744397.
Article37. Yeatman TJ, Risley GL, Brunson ME. Depletion of dietary arginine inhibits growth of metastatic tumor. Arch Surg. 1991; 126:1376–1381. PMID: 1747050.
Article38. Bach SJ, Lasnitzki I. Some aspects of the role of arginine and arginase in mouse carcinoma 63. Enzymologia. 1947; 12:198–205. PMID: 18910560.39. Storr JM, Burton AF. The effects of arginine deficiency on lymphoma cells. Br J Cancer. 1974; 30:50–59. PMID: 4528778.
Article40. Scott L, Lamb J, Smith S, Wheatley DN. Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br J Cancer. 2000; 83:800–810. PMID: 10952786.
Article41. Takaku H, Takase M, Abe S, Hayashi H, Miyazaki K. In vivo anti-tumor activity of arginine deiminase purified from Mycoplasma arginini. Int J Cancer. 1992; 51:244–249. PMID: 1568792.42. Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002; 62:5443–5450. PMID: 12359751.43. Bowles TL, Kim R, Galante J, Parsons CM, Virudachalam S, Kung HJ, et al. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008; 123:1950–1955. PMID: 18661517.
Article44. Hernandez CP, Morrow K, Lopez-Barcons LA, Zabaleta J, Sierra R, Velasco C, et al. Pegylated arginase I: a potential therapeutic approach in T-ALL. Blood. 2010; 115:5214–5221. PMID: 20407034.
Article45. Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, et al. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009; 69:700–708. PMID: 19147587.
Article46. Wu FL, Liang YF, Chang YC, Yo HH, Wei MF, Shen LJ. RNA interference of argininosuccinate synthetase restores sensitivity to recombinant arginine deiminase (rADI) in resistant cancer cells. J Biomed Sci. 2011; 18:25. PMID: 21453546.
Article47. Stelter L, Evans MJ, Jungbluth AA, Zanzonico P, Ritter G, Ku T, et al. Novel mechanistic insights into arginine deiminase pharmacology suggest 18F-FDG is not suitable to evaluate clinical response in melanoma. J Nucl Med. 2012; 53:281–286. PMID: 22228793.
Article48. Gong H, Zolzer F, von Recklinghausen G, Rossler J, Breit S, Havers W, et al. Arginine deiminase inhibits cell proliferation by arresting cell cycle and inducing apoptosis. Biochem Biophys Res Commun. 1999; 261:10–14. PMID: 10405315.
Article49. Gong H, Zolzer F, von Recklinghausen G, Havers W, Schweigerer L. Arginine deiminase inhibits proliferation of human leukemia cells more potently than asparaginase by inducing cell cycle arrest and apoptosis. Leukemia. 2000; 14:826–829. PMID: 10803513.
Article50. Morrow K, Hernandez CP, Raber P, Del Valle L, Wilk AM, Majumdar S, et al. Anti-leukemic mechanisms of pegylated arginase I in acute lymphoblastic T-cell leukemia. Leukemia. 2013; 27:569–577. PMID: 22926702.
Article51. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012; 149:274–293. PMID: 22500797.
Article52. Rhoads JM, Chen W, Gookin J, Wu GY, Fu Q, Blikslager AT, et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut. 2004; 53:514–522. PMID: 15016745.
Article53. Rhoads JM, Liu Y, Niu X, Surendran S, Wu G. Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J Nutr. 2008; 138:1652–1657. PMID: 18716165.
Article54. Fu YM, Zhang H, Ding M, Li YQ, Fu X, Yu ZX, et al. Specific amino acid restriction inhibits attachment and spreading of human melanoma via modulation of the integrin/focal adhesion kinase pathway and actin cytoskeleton remodeling. Clin Exp Metastasis. 2004; 21:587–598. PMID: 15787096.
Article55. Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, et al. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996; 379:466–469. PMID: 8559255.
Article56. Park IS, Kang SW, Shin YJ, Chae KY, Park MO, Kim MY, et al. Arginine deiminase: a potential inhibitor of angiogenesis and tumour growth. Br J Cancer. 2003; 89:907–914. PMID: 12942125.
Article57. Stelter L, Evans MJ, Jungbluth AA, Longo VA, Zanzonico P, Ritter G, et al. Imaging of tumor vascularization using fluorescence molecular tomography to monitor arginine deiminase treatment in melanoma. Mol Imaging. 2013; 12:67–73. PMID: 23348793.58. Gong H, Pottgen C, Stuben G, Havers W, Stuschke M, Schweigerer L. Arginine deiminase and other antiangiogenic agents inhibit unfavorable neuroblastoma growth: potentiation by irradiation. Int J Cancer. 2003; 106:723–728. PMID: 12866032.
Article59. Touz MC, Ropolo AS, Rivero MR, Vranych CV, Conrad JT, Svard SG, et al. Arginine deiminase has multiple regulatory roles in the biology of Giardia lamblia. J Cell Sci. 2008; 121(Pt 17):2930–2938. PMID: 18697833.
Article60. Morris SM Jr. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009; 157:922–930. PMID: 19508396.61. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998; 336(Pt 1):1–17. PMID: 9806879.
Article62. Leong HX, Simkevich C, Lesieur-Brooks A, Lau BW, Fugere C, Sabo E, et al. Short-term arginine deprivation results in large-scale modulation of hepatic gene expression in both normal and tumor cells: microarray bioinformatic analysis. Nutr Metab (Lond). 2006; 3:37. PMID: 16961918.
Article63. Brasse-Lagnel CG, Lavoinne AM, Husson AS. Amino acid regulation of mammalian gene expression in the intestine. Biochimie. 2010; 92:729–735. PMID: 20188788.
Article64. Shen LJ, Lin WC, Beloussow K, Shen WC. Resistance to the anti-proliferative activity of recombinant arginine deiminase in cell culture correlates with the endogenous enzyme, argininosuccinate synthetase. Cancer Lett. 2003; 191:165–170. PMID: 12618329.
Article65. Kim RH, Bold RJ, Kung HJ. ADI, autophagy and apoptosis: metabolic stress as a therapeutic option for prostate cancer. Autophagy. 2009; 5:567–568. PMID: 19276647.
Article66. Wang Z, Shi X, Li Y, Zeng X, Fan J, Sun Y, et al. Involvement of autophagy in recombinant human arginase-induced cell apoptosis and growth inhibition of malignant melanoma cells. Appl Microbiol Biotechnol. 2013; 8. 06. [Epub]. http://dx.doi.org/10.1007/s00253-013-5118-0 .
Article67. Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013; 497:633–637. PMID: 23665962.
Article68. Iwamoto S, Mihara K, Downing JR, Pui CH, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest. 2007; 117:1049–1057. PMID: 17380207.
Article69. Ehsanipour EA, Sheng X, Behan JW, Wang X, Butturini A, Avramis VI, et al. Adipocytes cause leukemia cell resistance to L-asparaginase via release of glutamine. Cancer Res. 2013; 73:2998–3006. PMID: 23585457.
Article70. Ellyard JI, Quah BJ, Simson L, Parish CR. Alternatively activated macrophage possess antitumor cytotoxicity that is induced by IL-4 and mediated by arginase-1. J Immunother. 2010; 33:443–452. PMID: 20463604.
Article71. Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005; 65:3044–3048. PMID: 15833831.
Article72. Kwong-Lam F, Chi-Fung CG. Vincristine could partly suppress stromal support to T-ALL blasts during pegylated arginase I treatment. Exp Hematol Oncol. 2013; 2:11. PMID: 23574711.
Article73. Phillips M, Szlosarek PW. Arginine metabolism and tumour-associated macrophages. In : Lawrence T, Hagemann T, editors. Tumour-associated macrophages. New York: Springer;2012. p. 77–90.74. Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH, et al. Pegylated recombinant human arginase (rhArgpeg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007; 67:309–317. PMID: 17210712.75. Tsai WB, Aiba I, Long Y, Lin HK, Feun L, Savaraj N, et al. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 2012; 72:2622–2633. PMID: 22461507.
Article76. You M, Savaraj N, Kuo MT, Wangpaichitr M, Varona-Santos J, Wu C, et al. TRAIL induces autophagic protein cleavage through caspase activation in melanoma cell lines under arginine deprivation. Mol Cell Biochem. 2013; 374:181–190. PMID: 23180246.
Article77. Vynnytska BO, Mayevska OM, Kurlishchuk YV, Bobak YP, Stasyk OV. Canavanine augments proapoptotic effects of arginine deprivation in cultured human cancer cells. Anticancer Drugs. 2011; 22:148–157. PMID: 20717004.
Article78. Vynnytska-Myronovska B, Bobak Y, Garbe Y, Dittfeld C, Stasyk O, Kunz-Schughart LA. Single amino acid arginine starvation efficiently sensitizes cancer cells to canavanine treatment and irradiation. Int J Cancer. 2012; 130:2164–2175. PMID: 21647872.
Article80. Deorukhkar A, Diep N, Chatterjee D, Diagardjane P, Bomalaski J, Krishnan S. Arginine deiminase: a novel radiosensitizer in pancreatic cancer in vitro and in vivo. In : 2014 ASCO Gastrointestinal Cancers Symposium; 2014 Jan 16-18; San Francisco, CA. Abstr no. 221.80. Daylami R, Muilenburg D, Bowles TL, Martinez SR, Bold RJ. Arginine deprivation by PEG-ADI induces autophagic cell death and enhances the tumor suppression effect of gemcitabine in pancreatic cancer. In : 2010 AACR 101st Annual Meeting; 2010 April 17-21; Washington, DC. Abstr no. 484.81. Li Y, Li X, Dai H, Sun X, Li J, Yang F, et al. Thymidylate synthase was associated with patient prognosis and the response to adjuvant therapy in bladder cancer. BJU Int. 2009; 103:547–552. PMID: 18990150.
Article82. Takezawa K, Okamoto I, Okamoto W, Takeda M, Sakai K, Tsukioka S, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer. 2011; 104:1594–1601. PMID: 21487406.
Article83. Righi L, Papotti MG, Ceppi P, Bille A, Bacillo E, Molinaro L, et al. Thymidylate synthase but not excision repair crosscomplementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol. 2010; 28:1534–1539. PMID: 20177021.
Article84. Sigmond J, Backus HH, Wouters D, Temmink OH, Jansen G, Peters GJ. Induction of resistance to the multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon cancer cells is associated with thymidylate synthase overexpression. Biochem Pharmacol. 2003; 66:431–438. PMID: 12907242.
Article85. Edler D, Kressner U, Ragnhammar P, Johnston PG, Magnusson I, Glimelius B, et al. Immunohistochemically detected thymidylate synthase in colorectal cancer: an independent prognostic factor of survival. Clin Cancer Res. 2000; 6:488–492. PMID: 10690528.86. Helleman J, Jansen MP, Span PN, van Staveren IL, Massuger LF, Meijer-van Gelder ME, et al. Molecular profiling of platinum resistant ovarian cancer. Int J Cancer. 2006; 118:1963–1971. PMID: 16287073.
Article87. Melaiu O, Cristaudo A, Melissari E, Di Russo M, Bonotti A, Bruno R, et al. A review of transcriptome studies combined with data mining reveals novel potential markers of malignant pleural mesothelioma. Mutat Res. 2012; 750:132–140. PMID: 22198210.
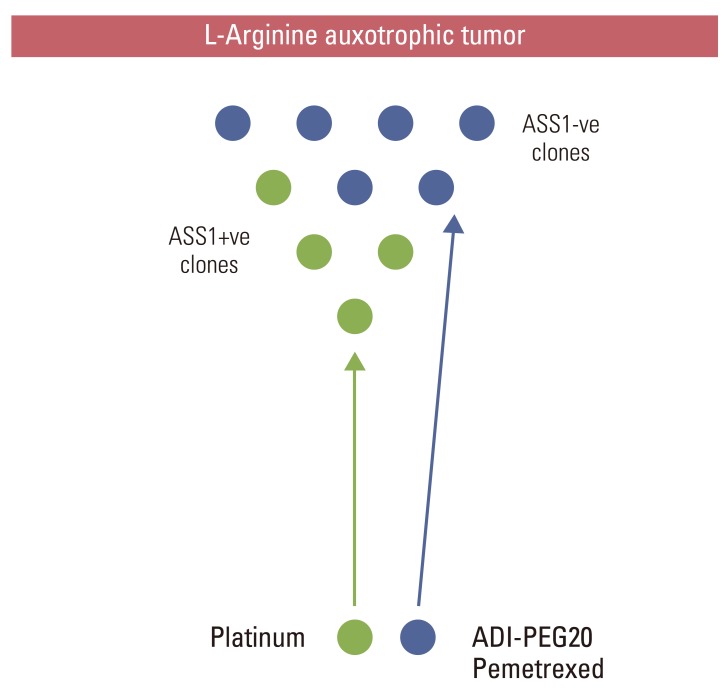
Article88. Nicholson LJ, Smith PR, Hiller L, Szlosarek PW, Kimberley C, Sehouli J, et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009; 125:1454–1463. PMID: 19533750.
Article89. Chow AK, Ng L, Sing Li H, Cheng CW, Lam CS, Yau TC, et al. Anti-tumor efficacy of a recombinant human arginase in human hepatocellular carcinoma. Curr Cancer Drug Targets. 2012; 12:1233–1243. PMID: 22873218.90. Savaraj N, You M, Wu C, Wangpaichitr M, Kuo MT, Feun LG. Arginine deprivation, autophagy, apoptosis (AAA) for the treatment of melanoma. Curr Mol Med. 2010; 10:405–412. PMID: 20459375.
Article91. Sikora AG, Gelbard A, Davies MA, Sano D, Ekmekcioglu S, Kwon J, et al. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin Cancer Res. 2010; 16:1834–1844. PMID: 20215556.
Article92. Grimm EA, Sikora AG, Ekmekcioglu S. Molecular pathways: inflammation-associated nitric-oxide production as a cancersupporting redox mechanism and a potential therapeutic target. Clin Cancer Res. 2013; 19:5557–5563. PMID: 23868870.
Article93. Stone E, Chantranupong L, Gonzalez C, O'Neal J, Rani M, VanDenBerg C, et al. Strategies for optimizing the serum persistence of engineered human arginase I for cancer therapy. J Control Release. 2012; 158:171–179. PMID: 22001609.
Article94. Agrawal V, Woo JH, Mauldin JP, Jo C, Stone EM, Georgiou G, et al. Cytotoxicity of human recombinant arginase I (Co)-PEG5000 in the presence of supplemental L-citrulline is dependent on decreased argininosuccinate synthetase expression in human cells. Anticancer Drugs. 2012; 23:51–64. PMID: 21955999.
Article95. Mauldin JP, Zeinali I, Kleypas K, Woo JH, Blackwood RS, Jo CH, et al. Recombinant human arginase toxicity in mice is reduced by citrulline supplementation. Transl Oncol. 2012; 5:26–31. PMID: 22348173.
Article96. Glazer ES, Stone EM, Zhu C, Massey KL, Hamir AN, Curley SA. Bioengineered human arginase I with enhanced activity and stability controls hepatocellular and pancreatic carcinoma xenografts. Transl Oncol. 2011; 4:138–146. PMID: 21633669.
Article97. Tanios R, Bekdash A, Kassab E, Stone E, Georgiou G, Frankel AE, et al. Human recombinant arginase I(Co)-PEG5000 [HuArgI(Co)-PEG5000]-induced arginine depletion is selectively cytotoxic to human acute myeloid leukemia cells. Leuk Res. 2013; 37:1565–1571. PMID: 24018014.
Article98. Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De Rosa V, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004; 22:1815–1822. PMID: 15143074.
Article99. Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005; 23:7660–7668. PMID: 16234528.
Article100. Glazer ES, Piccirillo M, Albino V, Di Giacomo R, Palaia R, Mastro AA, et al. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol. 2010; 28:2220–2226. PMID: 20351325.
Article101. Ott PA, Carvajal RD, Pandit-Taskar N, Jungbluth AA, Hoffman EW, Wu BW, et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Invest New Drugs. 2013; 31:425–434. PMID: 22864522.
Article102. Yang TS, Lu SN, Chao Y, Sheen IS, Lin CC, Wang TE, et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer. 2010; 103:954–960. PMID: 20808309.
Article103. Szlosarek PW, Steele JP, Sheaff MT, Avril NE, Szyszko T, Ellis S, et al. A randomised phase II trial of pegylated arginine deiminase (ADI-PEG20) in patients with malignant pleural mesothelioma (MPM). In : 2013 World Conference on Lung Cancer; 2013 Oct 27-30; Sydney. Abstr no. MO09.02.104. Yau T, Cheng PN, Chan P, Chan W, Chen L, Yuen J, et al. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2013; 31:99–107. PMID: 22426640.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Altered secretion of arginine vasopressin in children with CNS diseases
- Study on basic arginine amidase in human seminal plasma
- The mechanism of Arginine-stimulated growth hormone secretion
- Roles of Protein Arginine Methyltransferases in the Control of Glucose Metabolism
- Effect of Arginine Vasotocin on the Rabbit Renal Function