Cancer Res Treat.
2013 Sep;45(3):178-185.
How Molecular Understanding Affects to Prescribing Patterns and Clinical Outcome of Gefitinib in Non-small Cell Lung Cancer? 10 Year Experience of Single Institution
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. kimdw@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Gefitinib was introduced in 2002 for treatment of non-small cell lung cancer (NSCLC); however, it is not clear whether its use in daily practice has changed the outcome of patients. The purpose of this study is to evaluate the question of how molecular understanding regarding gefitinib and epidermal growth factor receptor (EGFR) mutation affect the prescribing patterns and clinical outcomes of treatment with gefitinib in NSCLC, in a real practical field.
MATERIALS AND METHODS
We conducted a retrospective analysis of the consecutive database of NSCLC patients who were treated with gefitinib at Seoul National University Hospital between January 2002 and December 2011. Prescribing patterns and clinical outcomes were analyzed by year.
RESULTS
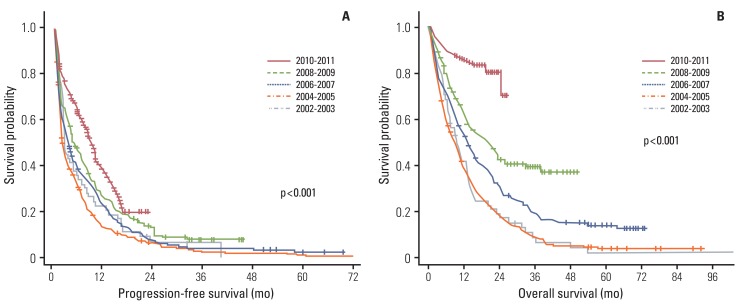
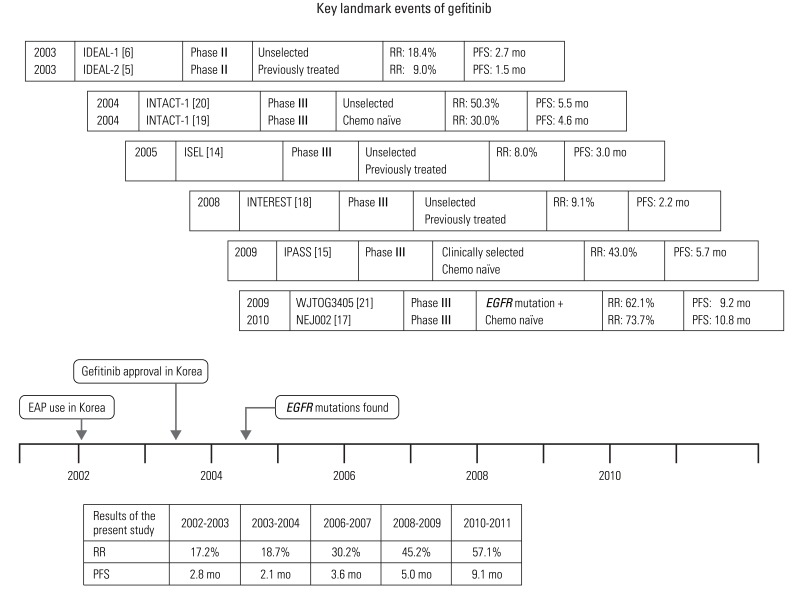
A total of 1,115 NSCLC patients, who received gefitinib at recurred or metastatic setting, were included in this study. Proportion of patients receiving gefitinib, for the first line, showed a gradual increase, from 5.2% in 2002-2003 to 30.6% in 2010-2011. Proportion of patients who underwent EGFR mutation testing showed a rapid increase, from 0.6% in 2004-2005 to 73.5% in 2010-2011. The response rate also showed a gradual increase, from 17.2% in 2002-2003 to 57.1% in 2010-2011 (p<0.001). The median progression-free survival of gefitinib was increased with statistical significance from 2.8 months in 2002-2003 to 9.1 months in 2010-2011 (p<0.001).
CONCLUSION
We demonstrated that molecular understanding and practical use of EGFR mutation testing have resulted in a change in the prescription patterns of gefitinib. Use of an enrichment strategy can lead to improvement in the efficacy of gefitinib in real practice.
Keyword
MeSH Terms
Figure
Reference
-
1. Lorusso PM. Phase I studies of ZD1839 in patients with common solid tumors. Semin Oncol. 2003; 30(1 Suppl 1):21–29. PMID: 12644981.
Article2. Ranson M, Hammond LA, Ferry D, Kris M, Tullo A, Murray PI, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002; 20:2240–2250. PMID: 11980995.
Article3. Herbst RS, Maddox AM, Rothenberg ML, Small EJ, Rubin EH, Baselga J, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002; 20:3815–3825. PMID: 12228201.
Article4. Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002; 20:4292–4302. PMID: 12409327.
Article5. Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003; 290:2149–2158. PMID: 14570950.6. Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003; 21:2237–2246. PMID: 12748244.7. Takano T, Ohe Y, Kusumoto M, Tateishi U, Yamamoto S, Nokihara H, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004; 45:93–104. PMID: 15196739.
Article8. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–2139. PMID: 15118073.
Article9. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004; 304:1497–1500. PMID: 15118125.10. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005; 23:2493–2501. PMID: 15710947.
Article11. Takano T, Ohe Y, Sakamoto H, Tsuta K, Matsuno Y, Tateishi U, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005; 23:6829–6837. PMID: 15998907.
Article12. Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005; 23:2513–2520. PMID: 15738541.
Article13. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004; 101:13306–13311. PMID: 15329413.
Article14. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005; 366:1527–1537. PMID: 16257339.
Article15. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–957. PMID: 19692680.
Article16. Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008; 26:4244–4252. PMID: 18779611.
Article17. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–2388. PMID: 20573926.
Article18. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008; 372:1809–1818. PMID: 19027483.
Article19. Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial: INTACT 2. J Clin Oncol. 2004; 22:785–794. PMID: 14990633.20. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004; 22:777–784. PMID: 14990632.
Article21. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010; 11:121–128. PMID: 20022809.
Article22. Kim YT, Kim TY, Lee DS, Park SJ, Park JY, Seo SJ, et al. Molecular changes of epidermal growth factor receptor (EGFR) and KRAS and their impact on the clinical outcomes in surgically resected adenocarcinoma of the lung. Lung Cancer. 2008; 59:111–118. PMID: 17904685.
Article23. Takano T, Fukui T, Ohe Y, Tsuta K, Yamamoto S, Nokihara H, et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol. 2008; 26:5589–5595. PMID: 18794545.24. Ahn MJ, Lee J, Park YH, Ahn JS, Ziogas A, Zell JA, et al. Korean ethnicity as compared with white ethnicity is an independent favorable prognostic factor for overall survival in non-small cell lung cancer before and after the oral epidermal growth factor receptor tyrosine kinase inhibitor era. J Thorac Oncol. 2010; 5:1185–1196. PMID: 20592628.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of leptomeningeal metastasis from adenocarcinoma of the lung improved by treatment with Gefitinib
- The Effectiveness of Gefitinib on Spinal Metastases of Lung Cancer: Report of Two Cases
- Inhibition of Ubiquitin-specific Peptidase 8 Suppresses Growth of Gefitinib-resistant Non-small Cell Lung Cancer Cells by Inducing Apoptosis
- Acute Respiratory Failure Developed in Non-small Cell Lung Cancer Patients Treated With Gefitinib
- Overcoming the Intrinsic Gefitinib-resistance via Downregulation of AXL in Non-small Cell Lung Cancer