Cancer Res Treat.
2013 Mar;45(1):63-69.
ERCC1 Can Be a Prognostic Factor in Hilar Cholangiocarcinoma and Extrahepatic Bile Duct Cancer, But Not in Intrahepatic Cholangiocarcinoma
- Affiliations
-
- 1Department of Internal Medicine, Seoul St. Mary's Hospital, Seoul, Korea. angelamd@catholic.ac.kr
- 2Department of Pathology, Seoul St. Mary's Hospital, Seoul, Korea.
- 3Department of Surgery, Seoul St. Mary's Hospital, Seoul, Korea.
- 4Department of Internal Medicine, Uijeongbu St. Mary's Hospital, Uijeongbu, Korea.
- 5Department of Internal Medicine, St. Vincent's Hospital, Suwon, The Catholic University of College of Medicine, Korea.
Abstract
- PURPOSE
There are three types of bile duct cancer, intrahepatic cholangiocarcinoma (ICC), hilar cholangiocarcinoma (HC), and extrahepatic cholangiocarcinoma (EHC). Despite different clinical presentation, the same protocol has been used in treatment of patients with these cancers. We analyzed clinicopathologic findings and protein expression in order to investigate the difference and the specific prognostic factors among these three types of cancers.
MATERIALS AND METHODS
We conducted a retrospective review of 104 patients diagnosed with bile duct cancer at Seoul St. Mary's Hospital between January 1994 and May 2004. We performed immunohistochemical staining for p53, cyclin D1, thymidine phosphorylase, survivin, and excision repair cross-complementing group 1 (ERCC1).
RESULTS
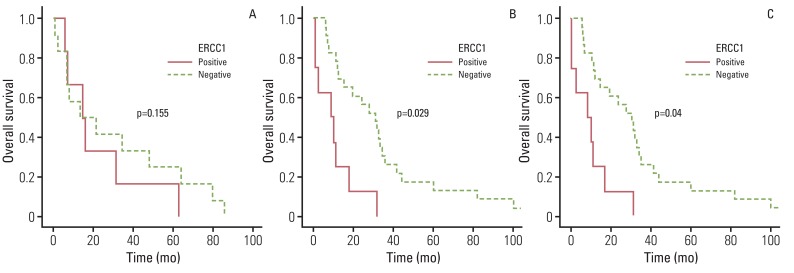
Of the 104 patients, EHC was most common (44.2%). In pathologic findings, perineural invasion was significantly less common in ICC. Overall survival was similar among the three types of cancer. Lymph node invasion, lymphatic, and venous invasion showed a significant association with survival outcome in ICC, however, the differentiation of histologic grade had prognostic significance in HC and EHC. No difference in protein expression was observed among these types of cancer, however, ERCC1 showed a significant association with survival outcome in HC and EHC, not in ICC.
CONCLUSION
Based on our data, ICC showed different characteristics and prognostic factors, separate from the other two types of bile duct cancer. Conduct of further studies with a large sample size is required in order to confirm these data.
Keyword
MeSH Terms
Figure
Reference
-
1. Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol. 2006; 45:856–867. PMID: 17030071.
Article2. Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001; 92:2374–2383. PMID: 11745293.3. Weber A, Landrock S, Schneider J, Stangl M, Neu B, Born P, et al. Long-term outcome and prognostic factors of patients with hilar cholangiocarcinoma. World J Gastroenterol. 2007; 13:1422–1426. PMID: 17457974.
Article4. Sano T, Shimada K, Sakamoto Y, Ojima H, Esaki M, Kosuge T. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol. 2008; 15:590–599. PMID: 18057991.
Article5. Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007; 31:1059–1067. PMID: 17592273.
Article6. Hong SM, Hwang I, Song DE, Choi J, Yu E. Clinical and prognostic significances of nuclear and cytoplasmic KIT expressions in extrahepatic bile duct carcinomas. Mod Pathol. 2007; 20:562–569. PMID: 17396144.
Article7. Arora DS, Ramsdale J, Lodge JP, Wyatt JI. p53 but not bcl-2 is expressed by most cholangiocarcinomas: a study of 28 cases. Histopathology. 1999; 34:497–501. PMID: 10383693.
Article8. Rashid A, Ueki T, Gao YT, Houlihan PS, Wallace C, Wang BS, et al. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002; 8:3156–3163. PMID: 12374683.9. Thanasai J, Limpaiboon T, Jearanaikoon P, Sripa B, Pairojkul C, Tantimavanich S, et al. Effects of thymidine phosphorylase on tumor aggressiveness and 5-fluorouracil sensitivity in cholangiocarcinoma. World J Gastroenterol. 2010; 16:1631–1638. PMID: 20355241.
Article10. Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996; 224:463–473. PMID: 8857851.11. Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990-2009. World J Gastroenterol. 2009; 15:4240–4262. PMID: 19750567.
Article12. Cereda S, Belli C, Reni M. Adjuvant treatment in biliary tract cancer: to treat or not to treat? World J Gastroenterol. 2012; 18:2591–2596. PMID: 22690066.
Article13. Shirai K, Ebata T, Oda K, Nishio H, Nagasaka T, Nimura Y, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2008; 32:2395–2402. PMID: 18795245.
Article14. Suzuki S, Sakaguchi T, Yokoi Y, Okamoto K, Kurachi K, Tsuchiya Y, et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg. 2002; 26:687–693. PMID: 12053220.
Article15. Tajima Y, Kuroki T, Fukuda K, Tsuneoka N, Furui J, Kanematsu T. An intraductal papillary component is associated with prolonged survival after hepatic resection for intrahepatic cholangiocarcinoma. Br J Surg. 2004; 91:99–104. PMID: 14716802.
Article16. Guedj N, Zhan Q, Perigny M, Rautou PE, Degos F, Belghiti J, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol. 2009; 51:93–101. PMID: 19446907.
Article17. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011; 29:3140–3145. PMID: 21730269.
Article18. Kim WB, Han HJ, Lee HJ, Park SS, Song TJ, Kim HK, et al. Expression and clinical significance of cell cycle regulatory proteins in gallbladder and extrahepatic bile duct cancer. Ann Surg Oncol. 2009; 16:23–34. PMID: 18979138.
Article19. Wang J, Wang X, Xie S, Yan Z, Li Z, Li Y, et al. p53 status and its prognostic role in extrahepatic bile duct cancer: a meta-analysis of published studies. Dig Dis Sci. 2011; 56:655–662. PMID: 20668938.
Article20. Jarnagin WR, Klimstra DS, Hezel M, Gonen M, Fong Y, Roggin K, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006; 24:1152–1160. PMID: 16505435.
Article21. Gossage L, Madhusudan S. Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev. 2007; 33:565–577. PMID: 17707593.
Article22. Toi M, Atiqur Rahman M, Bando H, Chow LW. Thymidine phosphorylase (platelet-derived endothelial-cell growth factor) in cancer biology and treatment. Lancet Oncol. 2005; 6:158–166. PMID: 15737832.
Article23. Aishima S, Taguchi K, Sugimachi K, Asayama Y, Nishi H, Shimada M, et al. The role of thymidine phosphorylase and thrombospondin-1 in angiogenesis and progression of intrahepatic cholangiocarcinoma. Int J Surg Pathol. 2002; 10:47–56. PMID: 11927969.
Article24. Javle MM, Tan D, Yu J, LeVea CM, Li F, Kuvshinoff BW, et al. Nuclear survivin expression predicts poor outcome in cholangiocarcinoma. Hepatogastroenterology. 2004; 51:1653–1657. PMID: 15532797.