Cancer Res Treat.
2012 Mar;44(1):37-42.
Chemotherapy in Patients Older than or Equal to 75 Years with Advanced Non-small Cell Lung Cancer
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Korea University School of Medicine, Seoul, Korea. shinsw@kumc.or.kr
Abstract
- PURPOSE
As the number of elderly patients diagnosed with non-small cell lung carcinoma (NSCLC) increases, the number of these patients receiving chemotherapy also increases. However, limited data exists regarding the use of chemotherapy in advanced NSCLC patients who are 75 years of age or older.
MATERIALS AND METHODS
Between May 2002 and October 2008, data for 48 advanced NSCLC patients who were 75 years of age or older who had been treated with chemotherapy were retrospectively analyzed.
RESULTS
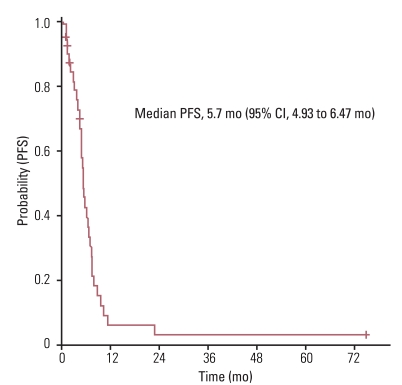
The median age of study participants at the time of first line chemotherapy was 76 years (range, 75 to 87 years) and their median Charlson comorbidity index was 2 (range, 1 to 4). Of the total 48 patients, 43 patients (90%) were treated by platinum-based doublet as a first line chemotherapy regimen. Median progression free survival for first line chemotherapy was 5.7 months (95% confidence interval [CI], 4.93 to 6.47 months) with an overall response rate of 33.3%. After first line chemotherapy, only 14 of the 48 patients (29.2%) received second line chemotherapy. The median overall survival (OS) for these patients was 8.2 months (95% CI, 4.44 to 11.96 months). Multivariate analysis results indicated that female gender and having received second-line or more chemotherapy were independent prognostic factors for increased OS for all 48 patients. Charlson Index was not a significant independent prognostic factor for survival. There were 9 treatment related deaths due to infectious causes (18.8%).
CONCLUSION
Patients 75 years of age or older with advanced NSCLC may obtain clinical benefit from the administration of platinum-based doublet or single agent chemotherapy. However, oncologists must consider the aspect of safety in relation to the clinical benefits when managing this patient group.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57:43–66. PMID: 17237035.
Article2. Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LA, Anderson RN, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003; 97(12 Suppl):3133–3275. PMID: 12784323.
Article3. Havlik RJ, Yancik R, Long S, Ries L, Edwards B. The National Institute on Aging and the National Cancer Institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer. 1994; 74(7 Suppl):2101–2106. PMID: 8087777.
Article4. Ruano-Ravina A, Pérez-Becerra R, Fraga M, Kelsey KT, Barros-Dios JM. Analysis of the relationship between p53 immunohistochemical expression and risk factors for lung cancer, with special emphasis on residential radon exposure. Ann Oncol. 2008; 19:109–114. PMID: 17897960.
Article5. Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003; 95:362–372. PMID: 12618501.
Article6. Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991; 9:1618–1626. PMID: 1651993.
Article7. Gridelli C, Aapro M, Ardizzoni A, Balducci L, De Marinis F, Kelly K, et al. Treatment of advanced non-small-cell lung cancer in the elderly: results of an international expert panel. J Clin Oncol. 2005; 23:3125–3137. PMID: 15860872.
Article8. Gridelli C, Langer C, Maione P, Rossi A, Schild SE. Lung cancer in the elderly. J Clin Oncol. 2007; 25:1898–1907. PMID: 17488989.
Article9. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999; 341:2061–2067. PMID: 10615079.
Article10. Davidoff AJ, Rapp T, Onukwugha E, Zuckerman IH, Hanna N, Pandya N, et al. Trends in disparities in receipt of adjuvant therapy for elderly stage III colon cancer patients: the role of the medical oncologist evaluation. Med Care. 2009; 47:1229–1236. PMID: 19786906.11. Zuckerman IH, Rapp T, Onukwugha E, Davidoff A, Choti MA, Gardner J, et al. Effect of age on survival benefit of adjuvant chemotherapy in elderly patients with Stage III colon cancer. J Am Geriatr Soc. 2009; 57:1403–1410. PMID: 19563521.
Article12. Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003; 21:1383–1389. PMID: 12663731.
Article13. Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005; 23:3112–3124. PMID: 15860871.
Article14. Langer CJ, Manola J, Bernardo P, Kugler JW, Bonomi P, Cella D, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst. 2002; 94:173–181. PMID: 11830607.
Article15. Sederholm C, Hillerdal G, Lamberg K, Kölbeck K, Dufmats M, Westberg R, et al. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced or metastatic non-small-cell lung cancer: the Swedish Lung Cancer Study Group. J Clin Oncol. 2005; 23:8380–8388. PMID: 16293868.
Article16. Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. 2007; 99:847–857. PMID: 17551145.
Article17. Rajeswaran A, Trojan A, Burnand B, Giannelli M. Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer. 2008; 59:1–11. PMID: 17720276.
Article18. Pallis AG, Polyzos A, Boukovinas I, Agelidou A, Lamvakas L, Tsiafaki X, et al. Pooled analysis of elderly patients with non-small cell lung cancer treated with front line docetaxel/gemcitabine regimen: the Hellenic Oncology Research Group experience. J Thorac Oncol. 2008; 3:505–510. PMID: 18449003.
Article19. Nakamura Y, Sekine I, Furuse K, Saijo N. Retrospective comparison of toxicity and efficacy in phase II trials of 3-h infusions of paclitaxel for patients 70 years of age or older and patients under 70 years of age. Cancer Chemother Pharmacol. 2000; 46:114–118. PMID: 10972480.
Article20. The Elderly Lung Cancer Vinorelbine Italian Study Group. Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999; 91:66–72. PMID: 9890172.21. Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, et al. Gemcitabine plus vinorelbine yields better survival outcome than vinorelbine alone in elderly patients with advanced non-small cell lung cancer. A Southern Italy Cooperative Oncology Group (SICOG) phase III trial. Lung Cancer. 2001; 34(Suppl 4):S65–S69. PMID: 11742706.
Article22. Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010; 28:2191–2197. PMID: 20351329.
Article23. Clinical practice guidelines for the treatment of unresectable non-small-cell lung cancer. Adopted on May 16, 1997 by the American Society of Clinical Oncology. J Clin Oncol. 1997; 15:2996–3018. PMID: 9256144.24. ESMO. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of non-small-cell lung cancer (NSCLC). Ann Oncol. 2001; 12:1049–1050. PMID: 11583180.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Locally Advanced Non-small Cell Lung Cancer
- Adjuvant Chemotherapy for Completely Resected Non-Small Cell Lung Cancer
- Cytotoxic Chemotherapy for Non-small Cell Lung Cancer
- Efficacy of Combination Chemotherapy with Paclitaxel and Cisplatin in Patients with Advanced Non-Small Cell Lung Cancer
- Combined Modality Therapy for Locally Advanced Non-Small Cell Lung Cancer