Cancer Res Treat.
2011 Dec;43(4):260-263.
Fatal Ifosfamide-Induced Metabolic Encephalopathy in Patients with Recurrent Epithelial Ovarian Cancer: Report of Two Cases
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. catgut1-0@hanmail.net
Abstract
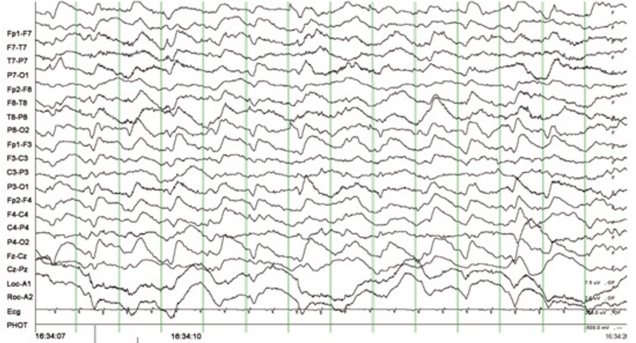
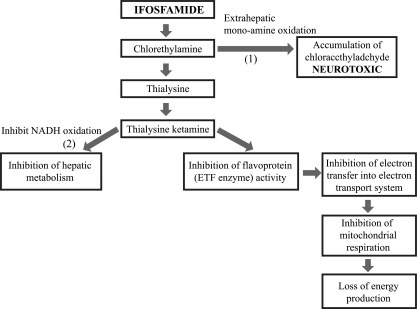
- Central nervous system (CNS) toxicity has been reported in approximately 10-30% of patients receiving intravenous infusions of ifosfamide. Encephalopathy is a rare but serious CNS adverse reaction in these patients, and although usually transient and reversible, may cause persistent neurological dysfunction or death. Clinical features range from fatigue and confusion to coma and death. Although methylene blue can be used to treat ifosfamide-induced neurotoxicity, including encephalopathy, its mechanism of action remains poorly defined. We describe here two patients with recurrent epithelial ovarian cancer who experienced fatal encephalopathy following ifosfamide/mesna treatment.
MeSH Terms
Figure
Reference
-
1. Giovanis P, Garna A, Marcante M, Nardi K, Giusto M. Ifosfamide encephalopathy and use of methylene blue: a case report of different sequential neurotoxicity. Tumori. 2009; 95:545–546. PMID: 19856674.
Article2. Ajithkumar T, Parkinson C, Shamshad F, Murray P. Ifosfamide encephalopathy. Clin Oncol (R Coll Radiol). 2007; 19:108–114. PMID: 17355105.
Article3. Alici-Evcimen Y, Breitbart WS. Ifosfamide neuropsychiatric toxicity in patients with cancer. Psychooncology. 2007; 16:956–960. PMID: 17278152.
Article4. Rieger C, Fiegl M, Tischer J, Ostermann H, Schiel X. Incidence and severity of ifosfamide-induced encephalopathy. Anticancer Drugs. 2004; 15:347–350. PMID: 15057138.
Article5. Meanwell CA, Blake AE, Kelly KA, Honigsberger L, Blackledge G. Prediction of ifosfamide/mesna associated encephalopathy. Eur J Cancer Clin Oncol. 1986; 22:815–819. PMID: 3095121.
Article6. Klastersky J. Side effects of ifosfamide. Oncology. 2003; 65(Suppl 2):7–10. PMID: 14586140.
Article7. McVay JI, Wood AM. Suspected ifosfamide-induced neurotoxicity. Pharmacotherapy. 1999; 19:1450–1455. PMID: 10600096.
Article8. Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prové A, Vermorken JB. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br J Cancer. 2000; 82:291–294. PMID: 10646879.
Article9. David KA, Picus J. Evaluating risk factors for the development of ifosfamide encephalopathy. Am J Clin Oncol. 2005; 28:277–280. PMID: 15923801.
Article10. Patel PN. Methylene blue for management of Ifosfamide-induced encephalopathy. Ann Pharmacother. 2006; 40:299–303. PMID: 16391008.
Article11. Küpfer A, Aeschlimann C, Cerny T. Methylene blue and the neurotoxic mechanisms of ifosfamide encephalopathy. Eur J Clin Pharmacol. 1996; 50:249–252. PMID: 8803513.
Article12. Turner AR, Duong CD, Good DJ. Methylene blue for the treatment and prophylaxis of ifosfamide-induced encephalopathy. Clin Oncol (R Coll Radiol). 2003; 15:435–439. PMID: 14570094.
Article13. Donegan S. Novel treatment for the management of ifosfamide neurotoxicity: rationale for the use of methylene blue. J Oncol Pharm Pract. 2001; 6:153–165.
Article14. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. 2003. Bethesda, MD: National Cancer Institute.15. Brunello A, Basso U, Rossi E, Stefani M, Ghiotto C, Marino D, et al. Ifosfamide-related encephalopathy in elderly patients: report of five cases and review of the literature. Drugs Aging. 2007; 24:967–973. PMID: 17953463.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Efficacy of Ifosfamide-Based Regimen in Refractory of Relapsed Ovarian Cancer
- A Clinical Study of Ifosfamide/Mesna and Cisplatin in Recurrent Epithelial Ovarian Cancer
- Selective Proximal Tubule Injury and Progressive Renal Failure due to Ifosfamide and Cisplatin in a Patient with Ovarian Cancer
- Ifosfamide-induced Fanconi syndrome with diabetes insipidus
- ATP-Based Chemotherapy Response Assay in Primary or Recurrent Ovarian and Peritoneal Cancer