Ann Surg Treat Res.
2014 Apr;86(4):192-198. 10.4174/astr.2014.86.4.192.
A multicenter experience with generic mycophenolate mofetil conversion in stable liver transplant recipients
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jw.joh@samsung.com
- 2Department of Surgery, Konkuk University School of Medicine, Seoul, Korea.
- 3Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Surgery, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2167125
- DOI: http://doi.org/10.4174/astr.2014.86.4.192
Abstract
- PURPOSE
Generic substitution of brand-name medications can lead to significant cost savings and is an accepted medical practice. This study evaluated clinical and safety outcomes among liver transplant recipients whose mycophenolate mofetil (MMF) was converted from the brand-name formulation (Cellcept) to a generic formulation (My-rept).
METHODS
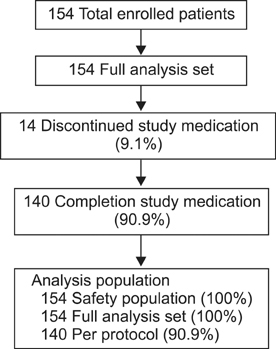
Clinical data from multiple centers were prospectively collected for determination of complications, safety, and quality of life after in 154 clinically stable, adult liver transplant recipients whose MMF was converted to a generic formulation between April 2010 and September 2012. This protocol was approved by Institutional Review Boards of all involved sites.
RESULTS
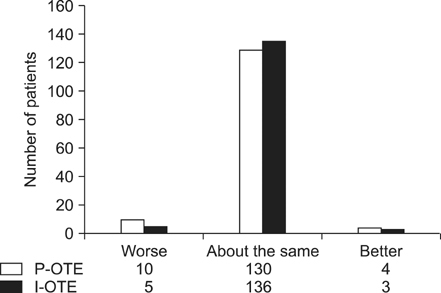
In eight patients (5.19%), nine instances of drug-related complications occurred after medication conversion. Half of these complications were gastrointestinal disorders (n = 4), and most (7 of 9) were mild. No significant differences were noted in mean pre- and postconversion gastrointestinal symptoms via a rating system (8.9 vs. 10.4) or gastrointestinal quality-of-life index scores (125.6 vs. 123.1). More than 90% of patients reported a status of "about the same" when questioned about the brand-name and generic formulation using the Patient Overall Treatment Effect and Investigator Overall Treatment Effect measures. The incidence of serious adverse events was 5.8%. Acute rejection occurred in two patients, with no graft loss or death.
CONCLUSION
Clinical experience as well as research data showed that generic MMF was comparable in efficacy to the brand-name drug. Given the lack of adverse events and the safety findings, conversion from brand-name MMF to generic MMF should be encouraged.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A cross-sectional analysis of long-term immunosuppressive regimens after liver transplantation at Asan Medical Center: Increased preference for mycophenolate mofetil
Shin Hwang, Chul-Soo Ahn, Ki-Hun Kim, Deok-Bog Moon, Tae-Yong Ha, Gi-Won Song, Dong-Hwan Jung, Gil-Chun Park, Sung-Gyu Lee
Ann Hepatobiliary Pancreat Surg. 2018;22(1):19-26. doi: 10.14701/ahbps.2018.22.1.19.
Reference
-
1. Sollinger HW. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation. 1995; 60:225–232.2. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996; 61:1029–1037.3. Ekberg H, Kyllonen L, Madsen S, Grave G, Solbu D, Holdaas H. Increased prevalence of gastrointestinal symptoms associated with impaired quality of life in renal transplant recipients. Transplantation. 2007; 83:282–289.4. Knoll GA, MacDonald I, Khan A, Van Walraven C. Mycophenolate mofetil dose reduction and the risk of acute rejection after renal transplantation. J Am Soc Nephrol. 2003; 14:2381–2386.5. Pelletier RP, Akin B, Henry ML, Bumgardner GL, Elkhammas EA, Rajab A, et al. The impact of mycophenolate mofetil dosing patterns on clinical outcome after renal transplantation. Clin Transplant. 2003; 17:200–205.6. Ensor CR, Trofe-Clark J, Gabardi S, McDevitt-Potter LM, Shullo MA. Generic maintenance immunosuppression in solid organ transplant recipients. Pharmacotherapy. 2011; 31:1111–1129.7. Harrison JJ, Schiff JR, Coursol CJ, Daley CJ, Dipchand AI, Heywood NM, et al. Generic immunosuppression in solid organ transplantation: a Canadian perspective. Transplantation. 2012; 93:657–665.8. Christians U. Generic immunosuppressants: the European perspective. Transplant Proc. 1999; 31:3A Suppl. 19S–22S.9. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997; 25:658–663.10. Kleinman L, Kilburg A, Machnicki G, Faull R, Walker R, Prasad R, et al. Using GI-specific patient outcome measures in renal transplant patients: validation of the GSRS and GIQLI. Qual Life Res. 2006; 15:1223–1232.11. Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmulling C, Neugebauer E, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995; 82:216–222.12. Guyatt G. Understanding the fundamentals of quality of life measurement. Evid Based Cardiovasc Med. 1998; 2:35–36.13. Kirking DM, Ascione FJ, Gaither CA, Welage LS. Economics and structure of the generic pharmaceutical industry. J Am Pharm Assoc (Wash). 2001; 41:578–584.14. Patel J, Kobashigawa JA. Minimization of immunosuppression: transplant immunology. Transpl Immunol. 2008; 20:48–54.15. Kuypers DR. Immunosuppressive drug monitoring - what to use in clinical practice today to improve renal graft outcome. Transpl Int. 2005; 18:140–150.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pretransplant mycophenolate mofetil reduces intrahepatic cholangiopathy related to laparoscopic donor hepatectomy in ABO-incompatible liver transplantation
- Phased Reduction of Cyclosporine Combined with Mycophenolate Mofetil in Renal Transplant Recipients: Three-year Results of a Prospective Study
- Single Center Experiences of Conversion from Twice-daily Tacrolimus (Prograf) to Once-daily Tacrolimus (Advagraf) in Stable Liver Transplant Recipients
- The Assessment of the Method of Monitoring in Liver Transplant Recipients Treated with Microemulsion Cyclosporine (Neoral(R)) and Mycophenolate Mofetil (CellCept(R))
- Prospective Controlled Protocol for Three Months Steroid Withdrawal with Tacrolimus, Basiliximab, and Mycophenolate Mofetil in Renal Transplant Recipients