Allergy Asthma Immunol Res.
2012 Sep;4(5):284-289. 10.4168/aair.2012.4.5.284.
Clinical Features and the Diagnostic Value of Component Allergen-Specific IgE in Hymenoptera Venom Allergy
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 3Department of Medicine, Gacheon University of Medicine and Science, Incheon, Korea.
- KMID: 2167047
- DOI: http://doi.org/10.4168/aair.2012.4.5.284
Abstract
- PURPOSE
Although patient history is vital for the diagnosis of hymenoptera venom allergy, specific IgE detection is also important to identify the culprit insect and monitor the effect of immunotherapy. We evaluated the diagnostic value of serum-specific IgE detection of hymenoptera venom component allergens and documented changes in allergen-specific IgE after immunotherapy.
METHODS
Fifty-six hymenoptera venom allergy patients receiving venom immunotherapy were recruited from Ajou University Hospital, Korea. The clinical manifestations of the patients were noted, and serum-specific IgE detection was performed, using conventional venom extracts as well as component allergens. Data were analyzed retrospectively.
RESULTS
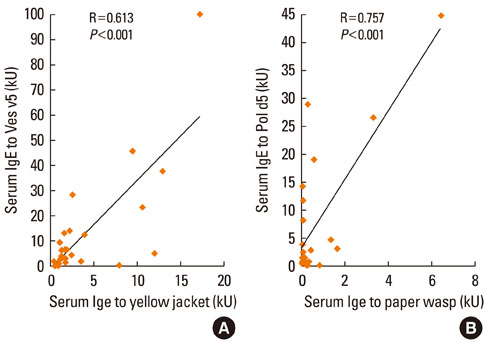
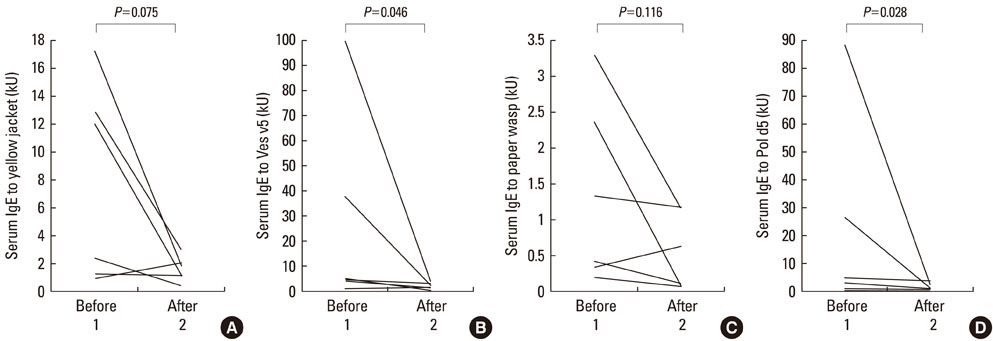
A total of 35 (62.5%) patients were male, and 33 (73.3%) patients were atopic. The mean patient age was 44.9+/-13.8 years. Localized reactions occurred in 23.2% of patients, and systemic reactions occurred in 76.8%. The most common clinical manifestations included skin involvement, such as urticaria and angioedema, and respiratory involvement. Yellow jackets were the most frequent culprit insect, followed by yellow hornets, white-faced hornets, honeybees, and paper wasps, as determined at the time of diagnosis. Double sensitization to both Apidae and Vespidae species was detected in 70.9% of patients. The positive predictive values (PPV) of rVes v 5-specific and rPol d 5-specific IgE detection were 85.7% and 87.5%, respectively, which correlated well with conventional venom extract-specific IgE detection (r=0.762 and r=0.757, respectively). In contrast, the PPV of rApi m 1-specific IgE detection at the time of diagnosis was 34.8%. Three years of venom immunotherapy resulted in decreased venom-specific IgE, particularly IgE specific for Vespidae venom components.
CONCLUSIONS
Stings by yellow jackets and male sex may be risk factors for hymenoptera venom allergy in Korea. Vespidae component-specific IgE, but not Apidae component-specific IgE, had diagnostic and monitoring value in hymenoptera venom allergy comparable to that of conventional hymenoptera venom extract-specific IgE.
MeSH Terms
Figure
Reference
-
1. Golden DB. Stinging insect allergy. Am Fam Physician. 2003. 67:2541–2546.2. Position paper: Immunotherapy with hymenoptera venoms. (EAACI) The European Academy of Allergology and Clinical Immunology. Allergy. 1993. 48:36–46.3. King TP, Sobotka AK, Kochoumian L, Lichtenstein LM. Allergens of honey bee venom. Arch Biochem Biophys. 1976. 172:661–671.4. Light WC, Reisman RE, Ilea VS, Wypych JI, Okazaki T, Arbesman CE. Studies of the antigenicity and allergenicity of phospholipase A2 of bee venom. J Allergy Clin Immunol. 1976. 58:322–329.5. Owen MD. Chemical components in the venoms of Ropalidia revolutionalis and Polistes humilis (Hymenoptera, Vespidae). Toxicon. 1979. 17:519–523.6. Egner W, Ward C, Brown DL, Ewan PW. The frequency and clinical significance of specific IgE to both wasp (Vespula) and honey-bee (Apis) venoms in the same patient. Clin Exp Allergy. 1998. 28:26–34.7. Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, Barletta B, Becker WM, Blaser K, Breiteneder H, Chapman M, Crameri R, Duchêne M, Ferreira F, Fiebig H, Hoffmann-Sommergruber K, King TP, Kleber-Janke T, Kurup VP, Lehrer SB, Lidholm J, Müller U, Pini C, Reese G, Scheiner O, Scheynius A, Shen HD, Spitzauer S, Suck R, Swoboda I, Thomas W, Tinghino R, Van Hage-Hamsten M, Virtanen T, Kraft D, Müller MW, Valenta R. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 2002. 16:414–416.8. Wöhrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, Stingl G, Kopp T. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006. 61:633–639.9. Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, Kraft D, Valenta R. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004. 34:597–603.10. Müller UR, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy. 2009. 64:543–548.11. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA Jr, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD Jr, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O'Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006. 47:373–380.12. Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004. 114:371–376.13. Björnsson E, Janson C, Plaschke P, Norrman E, Sjöberg O. Venom allergy in adult Swedes: a population study. Allergy. 1995. 50:800–805.14. Golden DB, Marsh DG, Kagey-Sobotka A, Freidhoff L, Szklo M, Valentine MD, Lichtenstein LM. Epidemiology of insect venom sensitivity. JAMA. 1989. 262:240–244.15. Shen Y, Li L, Grant J, Rubio A, Zhao Z, Zhang X, Zhou L, Fowler D. Anaphylactic deaths in Maryland (United States) and Shanghai (China): a review of forensic autopsy cases from 2004 to 2006. Forensic Sci Int. 2009. 186:1–5.16. Bonifazi F, Jutel M, Biló BM, Birnbaum J, Muller U. EAACI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy. 2005. 60:1459–1470.17. Akkoc T, Akdis M, Akdis CA. Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res. 2011. 3:11–20.18. Hemmer W, Focke M, Kolarich D, Wilson IB, Altmann F, Wöhrl S, Götz M, Jarisch R. Antibody binding to venom carbohydrates is a frequent cause for double positivity to honeybee and yellow jacket venom in patients with stinging-insect allergy. J Allergy Clin Immunol. 2001. 108:1045–1052.19. Blank S, Seismann H, Bockisch B, Braren I, Cifuentes L, McIntyre M, Rühl D, Ring J, Bredehorst R, Ollert MW, Grunwald T, Spillner E. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J Immunol. 2010. 184:5403–5413.20. Jin C, Focke M, Léonard R, Jarisch R, Altmann F, Hemmer W. Reassessing the role of hyaluronidase in yellow jacket venom allergy. J Allergy Clin Immunol. 2010. 125:184–190.e1.21. Hemmer W, Focke M, Kolarich D, Dalik I, Götz M, Jarisch R. Identification by immunoblot of venom glycoproteins displaying immunoglobulin E-binding N-glycans as cross-reactive allergens in honeybee and yellow jacket venom. Clin Exp Allergy. 2004. 34:460–469.22. Kochuyt AM, Van Hoeyveld EM, Stevens EA. Prevalence and clinical relevance of specific immunoglobulin E to pollen caused by sting-induced specific immunoglobulin E to cross-reacting carbohydrate determinants in Hymenoptera venoms. Clin Exp Allergy. 2005. 35:441–447.23. Bircher AJ, Van Melle G, Haller E, Curty B, Frei PC. IgE to food allergens are highly prevalent in patients allergic to pollens, with and without symptoms of food allergy. Clin Exp Allergy. 1994. 24:367–374.24. Lin J, Bardina L, Shreffler WG, Andreae DA, Ge Y, Wang J, Bruni FM, Fu Z, Han Y, Sampson HA. Development of a novel peptide microarray for large-scale epitope mapping of food allergens. J Allergy Clin Immunol. 2009. 124:315–322. 322.e1-e325. Sturm GJ, Jin C, Kranzelbinder B, Hemmer W, Sturm EM, Griesbacher A, Heinemann A, Vollmann J, Altmann F, Crailsheim K, Focke M, Aberer W. Inconsistent results of diagnostic tools hamper the differentiation between bee and vespid venom allergy. PLoS One. 2011. 6:e20842.26. de la Torre-Morin F, García-Robaina JC, Vázquez-Moncholí C, Fierro J, Bonnet-Moreno C. Epidemiology of allergic reactions in beekeepers: a lower prevalence in subjects with more than 5 years exposure. Allergol Immunopathol (Madr). 1995. 23:127–132.27. Annila IT, Karjalainen ES, Mörsky P, Kuusisto PA. Clinical symptoms and immunologic reactivity to bee and wasp stings in beekeepers. Allergy. 1995. 50:568–574.28. Hur GY, Kim JE, Ye YM, Suh CH, Nahm DH, Park HS. Clinical features of bee venom allergy. Korean J Asthma Allergy Clin Immunol. 2006. 26:145–150.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Past History on the Preceding Reaction to Bee Sting and Specific IgE Antibody to Bee Venom in Patients with Bee Sting Anaphylaxis
- Clinical Features of Bee Venom Allergy
- The Age Impact on Serum Total and Allergen-Specific IgE
- Detection of honeybee venom specific igE and igG4 in honeybee venom allergy
- In vitro Biphasic Effect of Honey Bee Venom on Basophils from Screened Healthy Blood Donors