Allergy Asthma Immunol Res.
2014 Jan;6(1):61-65. 10.4168/aair.2014.6.1.61.
Bioactive Lysophosphatidylcholine 16:0 and 18:0 Are Elevated in Lungs of Asthmatic Subjects
- Affiliations
-
- 1Department of Medicine, Rush University Medical Center, Chicago, IL, USA.
- 2Department of Pharmacology, Rush University Medical Center, Chicago, IL, USA. hlum@rush.edu
- 3Department of Immunology and Microbiology, Rush University Medical Center, Chicago, IL, USA.
- 4Department of Medicinal Chemistry & Pharmacognosy, University of Illinois, Chicago, IL, USA.
- KMID: 2166937
- DOI: http://doi.org/10.4168/aair.2014.6.1.61
Abstract
- PURPOSE
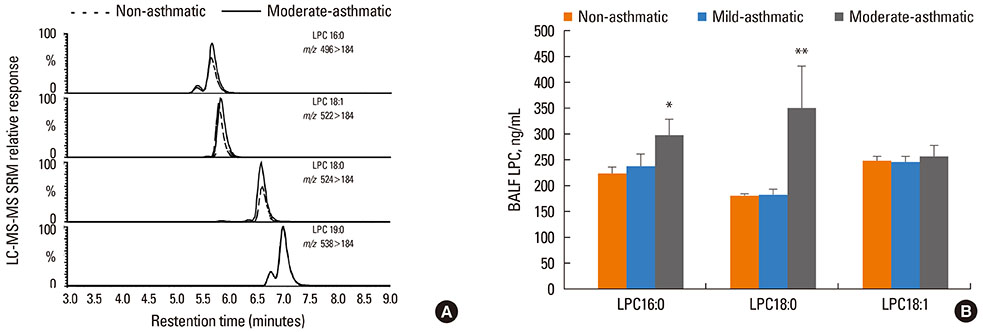
Asthma is a chronic inflammatory disease of the airways, and is associated with upregulation of phospholipase A2 (PLA2), the enzyme that hydrolyzes phosphatidylcholine, producing lysophosphatidylcholine (LPC) and free fatty acids. LPC is a lipid mediator with known pro-inflammatory and pro-atherogenic properties, and is believed to be a critical factor in cardiovascular diseases. We postulate that asthmatic subjects have an elevated content of LPC in the lung lining fluids.
METHODS
Eight non-asthmatic controls and seven asthmatic subjects were recruited for broncho-alveolar lavage fluids (BALF) collection for analysis of LPC by high performance liquid chromatography-tandem mass spectrometry.
RESULTS
LPC16:0 and LPC18:0 were significantly elevated in the BALF of asthmatics with impaired lung function characteristic of moderate asthma, but not mild asthma. The increased LPC content in BALF was accompanied by increased PLA2 activity. Furthermore, qRT-PCR analysis of the BALF cell fraction indicated increased secretory PLA2-X (sPLA2-X).
CONCLUSIONS
The increased LPC content in the lung lining fluids is a potential critical lipid mediator in the initiation and/or progression of airway epithelial injury in asthma.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Metabolomics strategies to discover new biomarkers associated to severe allergic phenotypes
Domingo Barber, Alma Villaseñor, Maria M. Escribese
Asia Pac Allergy. 2019;9(4):. doi: 10.5415/apallergy.2019.9.e37.
Reference
-
1. Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010; 208:10–18.2. Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem. 2007; 14:3209–3220.3. Ha CY, Kim JY, Paik JK, Kim OY, Paik YH, Lee EJ, Lee JH. The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 diabetes. Clin Endocrinol (Oxf). 2012; 76:674–682.4. Sasagawa T, Okita M, Murakami J, Kato T, Watanabe A. Abnormal serum lysophospholipids in multiple myeloma patients. Lipids. 1999; 34:17–21.5. Mehta D, Gupta S, Gaur SN, Gangal SV, Agrawal KP. Increased leukocyte phospholipase A2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. Am Rev Respir Dis. 1990; 142:157–161.6. Schaefer CA, Kuhlmann CR, Gast C, Weiterer S, Li F, Most AK, Neumann T, Backenköhler U, Tillmanns H, Waldecker B, Wiecha J, Erdogan A. Statins prevent oxidized low-density lipoprotein- and lysophosphatidylcholine-induced proliferation of human endothelial cells. Vascul Pharmacol. 2004; 41:67–73.7. Radu CG, Yang LV, Riedinger M, Au M, Witte ON. T cell chemotaxis to lysophosphatidylcholine through the G2A receptor. Proc Natl Acad Sci U S A. 2004; 101:245–250.8. Kohno M, Yokokawa K, Yasunari K, Minami M, Kano H, Hanehira T, Yoshikawa J. Induction by lysophosphatidylcholine, a major phospholipid component of atherogenic lipoproteins, of human coronary artery smooth muscle cell migration. Circulation. 1998; 98:353–359.9. Liu-Wu Y, Hurt-Camejo E, Wiklund O. Lysophosphatidylcholine induces the production of IL-1beta by human monocytes. Atherosclerosis. 1998; 137:351–357.10. Spangelo BL, Jarvis WD. Lysophosphatidylcholine stimulates interleukin-6 release from rat anterior pituitary cells in vitro. Endocrinology. 1996; 137:4419–4426.11. Takeshita S, Inoue N, Gao D, Rikitake Y, Kawashima S, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb. 2000; 7:238–246.12. Zou Y, Kim CH, Chung JH, Kim JY, Chung SW, Kim MK, Im DS, Lee J, Yu BP, Chung HY. Upregulation of endothelial adhesion molecules by lysophosphatidylcholine. Involvement of G protein-coupled receptor GPR4. FEBS J. 2007; 274:2573–2584.13. Nakos G, Kitsiouli EI, Tsangaris I, Lekka ME. Bronchoalveolar lavage fluid characteristics of early intermediate and late phases of ARDS. Alterations in leukocytes, proteins, PAF and surfactant components. Intensive Care Med. 1998; 24:296–303.14. Chilton FH, Averill FJ, Hubbard WC, Fonteh AN, Triggiani M, Liu MC. Antigen-induced generation of lyso-phospholipids in human airways. J Exp Med. 1996; 183:2235–2245.15. Arbibe L, Koumanov K, Vial D, Rougeot C, Faure G, Havet N, Longacre S, Vargaftig BB, Béréziat G, Voelker DR, Wolf C, Touqui L. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J Clin Invest. 1998; 102:1152–1160.16. Nishiyama O, Kume H, Kondo M, Ito Y, Ito M, Yamaki K. Role of lysophosphatidylcholine in eosinophil infiltration and resistance in airways. Clin Exp Pharmacol Physiol. 2004; 31:179–184.17. Nobata K, Kurashima K, Fujimura M, Abo M, Ishiura Y, Kasahara K, Nakao S. Inhaled lysophosphatidylcholine provokes bronchoconstriction in guinea pigs in vivo. Eur J Pharmacol. 2005; 520:150–155.18. Niewoehner DE, Rice K, Sinha AA, Wangensteen D. Injurious effects of lysophosphatidylcholine on barrier properties of alveolar epithelium. J Appl Physiol. 1987; 63:1979–1986.19. Lindahl M, Hede AR, Tagesson C. Lysophosphatidylcholine increases airway and capillary permeability in the isolated perfused rat lung. Exp Lung Res. 1986; 11:1–12.20. Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR Jr. Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2007; 176:1072–1078.21. Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997; 155:421–425.22. Triggiani M, Giannattasio G, Calabrese C, Loffredo S, Granata F, Fiorello A, Santini M, Gelb MH, Marone G. Lung mast cells are a source of secreted phospholipases A2. J Allergy Clin Immunol. 2009; 124:558–565. 565.e1–565.e3.23. Sane AC, Mendenhall T, Bass DA. Secretory phospholipase A2 activity is elevated in bronchoalveolar lavage fluid after ovalbumin sensitization of guinea pigs. J Leukoc Biol. 1996; 60:704–709.24. Chung YW, Oh HY, Kim JY, Kim JH, Kim IY. Allergen-induced proteolytic cleavage of annexin-1 and activation of cytosolic phospholipase A2 in the lungs of a mouse model of asthma. Proteomics. 2004; 4:3328–3334.25. Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009; 50:Suppl. S237–S242.26. Global Initiative for Asthma (GINA). GINA report. United States: Global Initiative for Asthma;2007. Available from: http://www.ginasthma.org.27. Zhu X, Learoyd J, Butt S, Zhu L, Usatyuk PV, Natarajan V, Munoz NM, Leff AR. Regulation of eosinophil adhesion by lysophosphatidylcholine via a non-store-operated Ca2+ channel. Am J Respir Cell Mol Biol. 2007; 36:585–593.28. Silliman CC, Elzi DJ, Ambruso DR, Musters RJ, Hamiel C, Harbeck RJ, Paterson AJ, Bjornsen AJ, Wyman TH, Kelher M, England KM, McLaughlin-Malaxecheberria N, Barnett CC, Aiboshi J, Bannerjee A. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 2003; 73:511–524.29. Huang F, Subbaiah PV, Holian O, Zhang J, Johnson A, Gertzberg N, Lum H. Lysophosphatidylcholine increases endothelial permeability: role of PKCalpha and RhoA cross talk. Am J Physiol Lung Cell Mol Physiol. 2005; 289:L176–L185.30. Huang F, Mehta D, Predescu S, Kim KS, Lum H. A novel lysophospholipid-and pH-sensitive receptor, GPR4, in brain endothelial cells regulates monocyte transmigration. Endothelium. 2007; 14:25–34.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of High-Resolution CT Findings between Asthmatic and Control Subjects
- Stimulated Bronchial Epithelial Cells Release Bioactive Lysophosphatidylcholine 16:0, 18:0, and 18:1
- Bronchodilator Response and Its Relationship to Bronchial Hyperresponsiveness in Children with Allergic Rhinitis/Asthma
- The Prevalence of Sensitivity to Sulfiting Agents in Patients with Bronchial Asthma
- Association between beta2 adrenoceptor polymorphisms and atopy/serum IgE in asthmatic patients