Allergy Asthma Immunol Res.
2016 Jan;8(1):55-62. 10.4168/aair.2016.8.1.55.
Effect of Amino Acid Polymorphisms of House Dust Mite Der p 2 Variants on Allergic Sensitization
- Affiliations
-
- 1Institute of Molecular Biosciences, Mahidol University, Salaya, Nakorn Pathom Thailand, Thailand. piboons@gmail.com
- 2Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand.
- 3Telethon Kids Institute, University of Western Australia, Perth, Australia.
- KMID: 2166650
- DOI: http://doi.org/10.4168/aair.2016.8.1.55
Abstract
- PURPOSE
The sequence variations of the Der p 2 allergen of Dermatophagoides pteronyssinus diverge along 2 pathways with particular amino acid substitutions at positions 40,47,111, and 114. The environmental prevalence and IgE binding to Der p 2 variants differ among regions. To compare IgE binding to Der p 2 variants between sera from Bangkok, Thailand and Perth, Western Australia with different variants and to determine the variant-specificity of antibodies induced by vaccination with recombinant variants.
METHODS
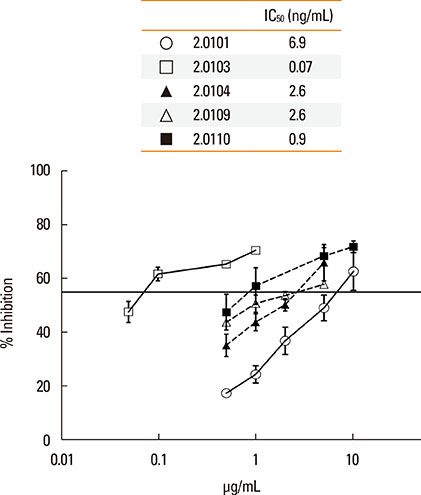
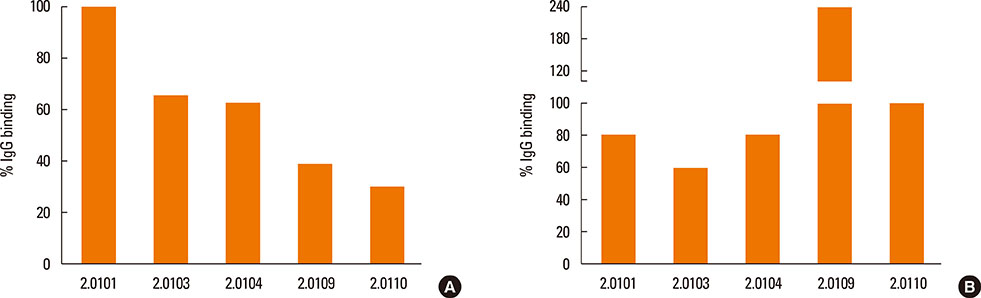
The structures of recombinant variants produced in yeast were compared by circular dichroism and 1-anilinonaphthalene 8-sulfonic acid staining of their lipid-binding cavity. Sera from subjects in Bangkok and Perth where different variants are found were compared by the affinity (IC50) of IgE cross-reactivity to different variants and by direct IgE binding. Mice were immunized with the variants Der p 2.0101 and Der p 2.0110, and their IgG binding to Der p 2.0103, 2.0104, and 2.0109 was measured.
RESULTS
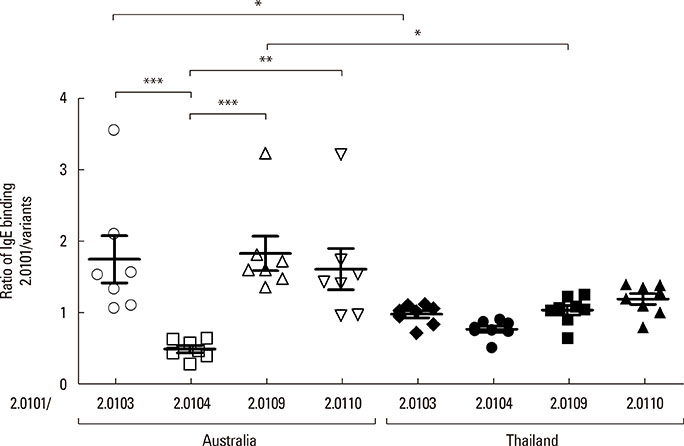
The secondary structures of the recombinant variants resembled the natural allergen but with differences in ANS binding. The IC50 of Der p 2.0101 required 7-fold higher concentrations to inhibit IgE binding to the high-IgE-binding Der p 2.0104 than for homologous inhibition in sera from Bangkok where it is absent, while in sera from Perth that have both variants the IC50 was the same and low. Reciprocal results were obtained for Der p 2.0110 not found in Perth. Direct binding revealed that Der p 2.0104 was best for detecting IgE in both regions, followed by Der p 2.0101 with binding to other variants showing larger differences. Mouse anti-Der p 2.0101 antibodies had a high affinity of cross-reactivity but bound poorly to other variants.
CONCLUSIONS
The affinity of IgE antibody cross-reactivity, the direct IgE binding, and the specificities of antibodies induced by vaccination show that measures of allergic sensitization and therapeutic strategies could be optimized with knowledge of Der p 2 variants.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Ser47-Point Mutation on Conformation Structure and Allergenicity of the Allergen of Der p 2, a Major House Dust Mite Allergen
Bhakkawarat Kulwanich, Sasipa Thanyaratsrisakul, Orathai Jirapongsananuruk, Belinda J. Hales, Wayne R. Thomas, Surapon Piboonpocanun
Allergy Asthma Immunol Res. 2019;11(1):129-142. doi: 10.4168/aair.2019.11.1.129.
Reference
-
1. Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010; 16:321–328.2. Inohara N, Nunez G. ML -- a conserved domain involved in innate immunity and lipid metabolism. Trends Biochem Sci. 2002; 27:219–221.3. Piboonpocanun S, Malainual N, Jirapongsananuruk O, Vichyanond P, Thomas WR. Genetic polymorphisms of major house dust mite allergens. Clin Exp Allergy. 2006; 36:510–516.4. Jeong KY, Lee IY, Yong TS, Lee JH, Kim EJ, Lee JS, et al. Sequence polymorphisms of Der f 1, Der p 1, Der f 2 and Der p 2 from Korean house dust mite isolates. Exp Appl Acarol. 2012; 58:35–42.5. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009; 457:585–588.6. Smith WA, Hales BJ, Jarnicki AG, Thomas WR. Allergens of wild house dust mites: environmental Der p 1 and Der p 2 sequence polymorphisms. J Allergy Clin Immunol. 2001; 107:985–992.7. Smith AM, Benjamin DC, Hozic N, Derewenda U, Smith WA, Thomas WR, et al. The molecular basis of antigenic cross-reactivity between the group 2 mite allergens. J Allergy Clin Immunol. 2001; 107:977–984.8. Hales BJ, Hazell LA, Smith W, Thomas WR. Genetic variation of Der p 2 allergens: effects on T cell responses and immunoglobulin E binding. Clin Exp Allergy. 2002; 32:1461–1467.9. Park JW, Kim KS, Jin HS, Kim CW, Kang DB, Choi SY, et al. Der p 2 isoallergens have different allergenicity, and quantification with 2-site ELISA using monoclonal antibodies is influenced by the isoallergens. Clin Exp Allergy. 2002; 32:1042–1047.10. Tanyaratsrisakul S, Malainual N, Jirapongsananuruk O, Smith WA, Thomas WR, Piboonpocanun S. Structural and IgE binding analyses of recombinant Der p 2 expressed from the hosts Escherichia coli and Pichia pastoris. Int Arch Allergy Immunol. 2010; 151:190–198.11. Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995; 4:2411–2423.12. Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000; 287:252–260.13. Hakkaart GA, Chapman MD, Aalberse RC, van Ree R. Immune-reactivity of recombinant isoforms of the major house dust mite allergen Der p 2. Clin Exp Allergy. 1998; 28:169–174.14. Ichikawa S, Takai T, Inoue T, Yuuki T, Okumura Y, Ogura K, et al. NMR study on the major mite allergen Der f 2: its refined tertiary structure, epitopes for monoclonal antibodies and characteristics shared by ML protein group members. J Biochem. 2005; 137:255–263.15. Mueller GA, Smith AM, Williams DC Jr, Hakkaart GA, Aalberse RC, Chapman MD, et al. Expression and secondary structure determination by NMR methods of the major house dust mite allergen Der p 2. J Biol Chem. 1997; 272:26893–26898.16. Malainual N, Vichyanond P, Phan-Urai P. House dust mite fauna in Thailand. Clin Exp Allergy. 1995; 25:554–560.17. Christensen LH, Riise E, Bang L, Zhang C, Lund K. Isoallergen variations contribute to the overall complexity of effector cell degranulation: effect mediated through differentiated IgE affinity. J Immunol. 2010; 184:4966–4972.18. Thomas WR. Geography of house dust mite allergens. Asian Pac J Allergy Immunol. 2010; 28:211–224.19. Resch Y, Weghofer M, Seiberler S, Horak F, Scheiblhofer S, Linhart B, et al. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy. 2011; 41:1468–1477.20. Hales BJ, Laing IA, Pearce LJ, Hazell LA, Mills KL, Chua KY, et al. Distinctive immunoglobulin E anti-house dust allergen-binding specificities in a tropical Australian Aboriginal community. Clin Exp Allergy. 2007; 37:1357–1363.21. Banerjee S, Resch Y, Chen KW, Swoboda I, Focke-Tejkl M, Blatt K, et al. Der p 11 is a major allergen for house dust mite-allergic patients suffering from atopic dermatitis. J Invest Dermatol. 2015; 135:102–109.22. Vrtala S, Huber H, Thomas WR. Recombinant house dust mite allergens. Methods. 2014; 66:67–74.23. O'Hehir RE, Verhoef A, Panagiotopoulou E, Keswani S, Hayball JD, Thomas WR, et al. Analysis of human T cell responses to the group II allergen of Dermatophagoides species: localization of major antigenic sites. J Allergy Clin Immunol. 1993; 92:105–113.24. Walgraffe D, Mattéotti C, El Bakkoury M, Garcia L, Marchand C, Bullens D, et al. A hypoallergenic variant of Der p 1 as a candidate for mite allergy vaccines. J Allergy Clin Immunol. 2009; 123:1150–1156.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- House Dust Endotoxin Exposure and Allergic Sensitization in Korean Home Living Conditions
- Concentrations of Dust Mite in The Dust of Childhood Bedclothing, Cloth Wrappers, and Sewing Dolls
- House Dust Mite Allergic Rhinitis Model in C57BL/6 Mice
- Sensitization of house dust mites in the allergic patients and mite ecology in their house dusts
- Correlation between House Dust Mite Allergen Concentrations in Scalp Dander and Clinical Severity of Atopic Dermatitis in Children