Cancer Res Treat.
2004 Jun;36(3):173-177.
Combination of Gemcitabine and Cisplatin as First-Line Therapy in Advanced Non-Small-Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Soon Chun Hyang University College of Medicine, Seoul, Korea. parkhs@hosp.sch.ac.kr
- 2Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The prognosis of patients with advanced non-small-cell lung cancer (NSCLC) is extremely poor. Many prospective randomized trials on patients with advanced NSCLC suggested systemic chemotherapy improves both the survival and quality of life. A phase II trial was conducted to evaluate the efficacy and safety profile of the combination chemotherapy of gemcitabine and cisplatin in advanced NSCLC.
MATERIALS AND METHODS
Forty-four patients with locally advanced or metastatic NSCLC were enrolled. The patients received a cisplatin, 75 mg/m(2), infusion over 30 minutes on days 1, followed by a gemcitabine, 1, 250 mg/m(2), infusion over 30 minutes on days 1 and 8 every 3 weeks.
RESULTS
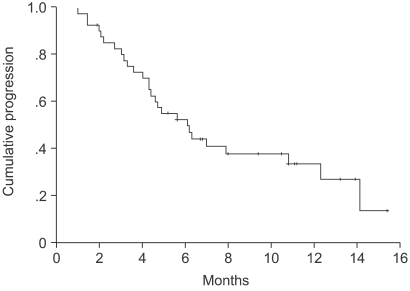
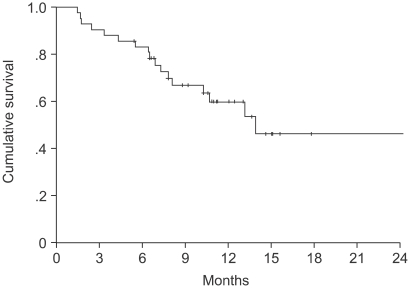
The median age of the patients was 64 years (range: 27~75). Forty-one patients were assessable for response and toxicity analyses. The overall response rate was 53.6%, but with no complete remissions. The median time to progression was 5.6 months (range: 1~15.4). The median survival was 14.2 months (95% confidence interval (CI), 13.8~22.5). A total of 179 cycles were administered, with a median of 4 cycles of chemotherapy, ranging from 2 to 9 cycles. The most common hematological toxicities were NCI grades 3/4 neutropenia (24%) and thrombocytopenia (7.8%). The most common non-hematological toxicity was fatigue (42.4%). There were no life-threatening toxicity or treatment related mortalities. The median duration of follow up was 9.4 months, ranging from 1.6 to 30.3 months.
CONCLUSION
In this trial, the combination of gemcitabine and cisplatin showed significant activity, with acceptable and manageable toxicities as a first-line regimen for patients with advanced NSCLC.
MeSH Terms
Figure
Reference
-
1. Vokes EE, Bitran JD, Vogelzang NJ. Chemotherapy for non-small-cell lung cancer. The continuing challenge. Chest. 1991; 99:1326–1328. PMID: 1645242.2. Ginsberg RJ, Vokes EE, Rosenzweig K. De Vita VT, Hellman S, Rosenberg SR, editors. Non small cell lung cancer. Cancer: Principles and Practice of Oncology. 2001. Vol 1:6th ed. Philadelphia: Lippincott-Raven;p. 925–983.3. Bunn PA Jr, Kelly K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: A review of the literature and future directions. Clin Cancer Res. 1998; 4:1087–1100. PMID: 9607565.4. Marino P, Pampallona S, Preatoni A, Cantoni A, Invernizzi F. Chemotherapy vs. supportive care in advanced non-small-cell lung cancer: results of a meta-analysis of the literature. Chest. 1994; 106:861–865. PMID: 7521815.5. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995; 311:899–909. PMID: 7580546.6. Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small cell lung cancer: how much benefit is enough? J Clin Oncol. 1993; 11:1866–1872. PMID: 8410111.7. Cullen MH, Billingham LJ, Woodroffe CM, Chetiyawardana AD, Gower NH, Joshi R, Ferry DR, Rudd RM, Spiro SG, Cook JE, Trask C, Bessell E, Connolly CK, Tobias J, Souhami RL. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: effects on survival and quality of life. J Clin Oncol. 1999; 17:3188–3194. PMID: 10506617.
Article9. Bunn PA Jr. The treatment of non-small cell lung cancer: Current perspectives and controversies, future directions. Semin Oncol. 1994; 21:49–59. PMID: 8052874.10. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98. PMID: 11784875.
Article11. Barton BM. Gemcitabine: A pharmacologic and clinical overview. Cancer Nurs. 1999; 22:176–183. PMID: 10217035.12. Shepherd FA. Phase II trials of single-agent activity of gemcitabine in patients with advanced non-small cell lung cancer: An overview. Anticancer Drugs. 1995; 6:19–25. PMID: 8718421.
Article13. Gatzemeier U, Shepherd FA, Le Chevalier T, Weynants P, Cottier B, Groen HJ, Rosso R, Mattson K, Cortes-Funes H, Tonato M, Burkes RL, Gottfried M, Voi M. Activity of gemcitabine in patients with non-small cell lung cancer: A multicentre extended phase II study. Eur J Cancer. 1996; 32:243–248. PMID: 8664035.
Article14. Chang HJ, Ahn JB, Lee JG, Shim KY, Rha SY, Kim SK, Chang J, Kim SK, Lee WY, Yoo NC, Chung HC, Roh JK, Kim BS, Choi SJ, Kim TW, Suh CW, Kim JH. Efficacy of gemcitabine chemotherapy in advanced non-small cell lung cancer (NSCLC): a phase II study. J Korean Cancer Assoc. 1997; 31:523–532.15. Crino L, Scagliotti G, Marangolo M, Figoli F, Clerici M, De Marinis F, Salvati F, Cruciani G, Dogliotti L, Pucci F, Paccagnella A, Adamo V, Altavilla G, Incoronato P, Trippetti M, Mosconi AM, Santucci A, Sorbolini S, Oliva C, Tonato M. Cisplatin-gemcitabine combination in advanced non-small-cell lung cancer: A phase II study. J Clin Oncol. 1997; 15:297–303. PMID: 8996156.16. Abratt RP, Bezwoda WR, Goedhals L, Hacking DJ. Weekly gemcitabine and monthly cisplatin: Effective chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 1997; 15:744–749. PMID: 9053500.17. Rossi D, Graziano F, Catalano V, Giordani P, Fedeli SL, Alessandroni P, Fedeli A, Dennetta D, Ugolini M, Catalano G. A new cisplatin/gemcitabine schedule in locally advanced (IIIB) and metastatic (IV) non-small cell lung cancer: relationship between dose-intensity and efficacy: A phase II study. Anticancer Res. 2002; 22:3087–3092. PMID: 12530048.18. Bretti S, Manzin E, Loddo C, Berruti A, Bombaci S, Vellani G, Celano A. Gemcitabine plus cisplatin in the treatment of patients with advanced non-small cell lung cancer: a phase II study. Anticancer Res. 2002; 22:3039–3043. PMID: 12530039.19. Kusaba H, Tamura T, Shimoyama T, Hotta K, Inoue A, Nokihara H, Ueda Y, Akiyama Y, Yamamoto N, Sekine I, Kunitoh H, Ohe Y, Kodama T, Saijo N. Phase I/II study of 3-week cycle cisplatin-gemcitabine in advanced non-small cell lung cancer. Jpn J Clin Oncol. 2002; 32:43–47. PMID: 11948227.20. Jassem J, Krzakowski M, Roszkowski K, Ramlau R, Slominski JM, Szczesna A, Krawczyk K, Mozejko-Pastewka B, Lis J, Miracki K. A phase II study of gemcitabine plus cisplatin in patients with advanced non-small cell lung cancer: clinical outcomes and quality of life. Lung Cancer. 2002; 351:73–79. PMID: 11750716.
Article21. Rinaldi M, Crino L, Scagliotti GV, Mosconi AM, De Marinis F, Gridelli C, Selvaggi G, Della Giulia M, Darwish S, Porrozzi S, Novello S, Cipri A, Bartolucci R, Calandri C, Tonato M. A three-week schedule of gemcitabine-cisplatin in advanced non-small-cell lung cancer with two different cisplatin dose levels: a phase II randomized trial. Ann Oncol. 2000; 11:1295–1300. PMID: 11106119.
Article22. Kim SH, Lee GW, Yoon JH, Shim KS, Lee YM, Kang DY, Park JR, Jung JH, Shin MK, Jeong YY, Kim HC, Lee WS, Lee JD, Hwang YS, Lee JS, Jang JS. 3-week-scheduled combination chemotherapy of gemcitabine and cisplatin in patients with advanced NSCLC. Korean J Med. 2004; 66:58–66.23. Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F, Smit H, Gaafar R, Biesma B, Manegold C, Neymark N, Giaccone G. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group-EORTC 08975. J Clin Oncol. 2003; 21:3909–3917. PMID: 14581415.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Combination Chemotherapy with Paclitaxel and Cisplatin in Patients with Advanced Non-Small Cell Lung Cancer
- Enhancement of Cytotoxicity by the Combination of Anticancer Drugs in Human Lung Adenocarcinoma Cell Line (PC-14)
- Gemcitabine/Cisplatin Combination Chemotherapy in Advanced non-Small Cell lung Cancer
- Treatment of Advanced and Metastatic Squamous Non-small Cell Lung Cancer
- A Comparison of Gemcitabine in Two Doses for Stage III or IV Non-small Cell Lung Cancer : a Multi-Institutional Phase II Study