Cancer Res Treat.
2004 Aug;36(4):255-262.
Comparison of As2O3 and As4O6 in the Detection of SiHa Cervical Cancer Cell Growth Inhibition Pathway
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul, Korea. ahnws@catholic.ac.kr
- 2Catholic Research Institutes of Medical Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3College of Pharmacy, Seoul National University, Seoul, Korea.
- 4Department of Biochemistry and Molecular Biology, Seoul National University, Seoul, Korea.
- 5Department of Medicine, Catholic University of Daegu, Daegu, Korea.
Abstract
- PURPOSE
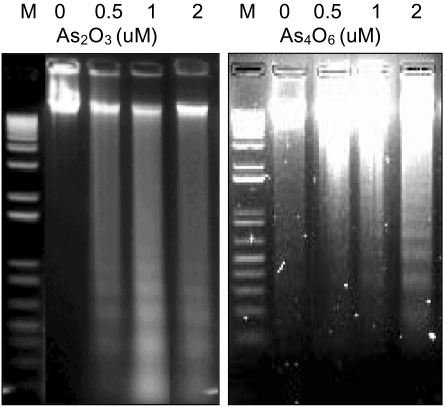
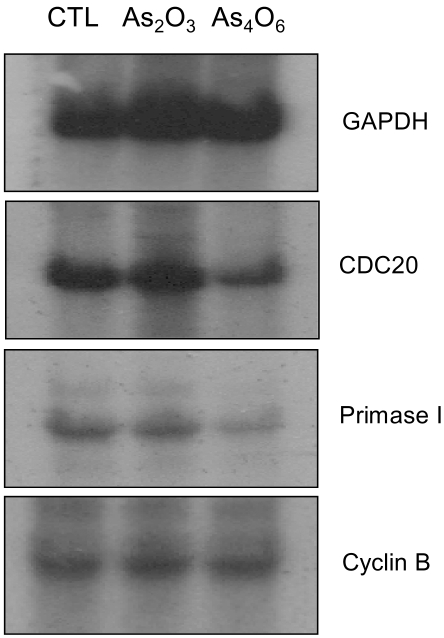
An arsenical compound, As2O3, has been reported to be effective for treating acute leukemia and inducing apoptosis in many different tumor cells. In this study, the ability of As4O6 to suppress cell growth and induce gene expression patterns was tested using a cDNA microarray in HPV16 immortalized cervical carcinoma cells, SiHa cells, along with As2O3. MATERIALS AND METHODS: A novel arsenical compound, As4O6, was designed and its ability to induce cell growth inhibition as well as gene expression profiles along with As2O3 in HPV16 infected SiHa cervical cancer cells was compared. Both As2O3 and As4O6 induced apoptosis in SiHa cells, as determined by DNA ladder formation. To further compare the gene expression profiles between these two drugs, a 384 cDNA microarray system was employed. Also, the gene expression profiles were classified into the Gene Ontology (GO) to investigate apoptosis-related cellular processes. RESULTS: As4O6 was more effective i suppressing the growth of SiHa cells in vitro compared to As2O3. In the case of treatment with As2O3, 41 genes were up- or down- regulated at least 2 fold compared to non-treatment. However, 65 genes were up- or down-regulated by As4O6 treatment. In particular, 27 genes were commonly regulated by both arsenic compounds. Also, the GO analysis indicated that down-regulation of cell-regulatory functions, such as cell cycle, protein kinase activity and DNA repair, induced anti-tumor effect. CONCLUSION: These data support that As4O6 could be more effective than As2O3 in inhibiting the growth of HPV16 infected cervical cancer cells. This appears to be mediated through a unique, but overlapping regulatory mechanism(s), suggesting that the regulated genes and cellular processes could be further used as a new potential drug approach for treating cervical cancer in clinical settings.
MeSH Terms
Figure
Reference
-
1. Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti L, Corso D, Deblasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP Jr. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998; 339:1341–1348. PMID: 9801394.
Article2. Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, Kim BK, Lee YY. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000; 60:3065–3071. PMID: 10850458.3. Gurr JR, Bau DT, Liu F, Lynn S, Jan KY. Dithiothreitol enhances arsenic trioxide-induced apoptosis in NB4 cells. Mol Pharmacol. 1999; 56:102–109. PMID: 10385689.
Article4. Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA-immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer. 1999; 82:286–292. PMID: 10389765.
Article5. Yih LH, Lee TC. Arsenite induces p53 accumulation through an ATM-dependent pathway in human fibroblasts. Cancer Res. 2000; 60:6346–6352. PMID: 11103796.6. St. John LS, Sauter ER, Herlyn M, Litwin S, Adler-Storthz K. Endogenous p53 gene status predicts the response of human squamous cell carcinomas to wild-type p53. Cancer Gene Ther. 2000; 7:749–756. PMID: 10830722.
Article7. Dong JT, Luo XM. Arsenic-induced DNA-strand breaks associated with DNA-protein crosslinks in human fetal lung fibroblasts. Mutat Res. 1993; 302:97–102. PMID: 7684511.
Article8. Simeonova PP, Wang S, Toriuma W, Kommineni V, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster MI. Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with activating protein-1 transactivation. Cancer Res. 2000; 60:3445–3453. PMID: 10910055.9. Buzard GS, Kasprzak KS. Possible roles of nitric oxide and redox cell signaling in metal-induced toxicity and carcinogenesis: a review. J Environ Pathol Toxicol Oncol. 2000; 19:179–199. PMID: 10983886.10. Lorincz AT, Temple GF, Kurman RJ, Jenson AB, Lancaster WD. Oncogenic association of specific human papillomavirus types with cervical neoplasia. J Natl Cancer Inst. 1987; 79:671–677. PMID: 2821311.11. Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990; 248:76–79. PMID: 2157286.
Article12. Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol. 2002; 12:712–723. PMID: 12007414.
Article13. Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999; 93:268–277. PMID: 9864170.
Article14. Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the national cancer institute cooperative research and development studies. Oncologist. 2001; 6:22–28. PMID: 11331437.
Article15. Ermolaeva O, Rastogi M, Pruitt KD, Schuler GD, Bittner ML, Chen Y, Simon R, Meltzer P, Trent JM, Boguski MS. Data management and analysis for gene expression arrays. Nat Genet. 1998; 20:19–23. PMID: 9731524.
Article16. Scherf U, Ross DT, Waltham M, Smith LH, Lee JK, Tanabe L, Kohn KW, Reinhold WC, Myers TG, Andrews DT, Scudiero DA, Eisen MB, Sausville EA, Pommier Y, Botstein D, Brown PO, Weinstein JN. A gene expression database for the molecular pharmacology of cancer. Nat Genet. 2000; 24:236–244. PMID: 10700175.
Article17. Lu T, Liu J, LeCluyse EL, Zhou YS, Cheng ML, Waalkes MP. Application of cDNA microarray to the study of arsenic-induced liver diseases in the population of Guizhou, China. Toxicol Sci. 2001; 59:185–192. PMID: 11134558.
Article18. Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999; 1:1349–1365. PMID: 10611762.19. Nowak RA. Fibroids: pathophysiology and current medical treatment. Baillieres Best Pract Res Clin Obstet Gynaecol. 1999; 13:223–238. PMID: 10755039.
Article20. Metcalfe A, Streuli C. Epithelial apoptosis. Bioessays. 1997; 19:711–720. PMID: 9264254.
Article21. Dynlacht BD. Regulation of transcription by proteins that control the cell cycle. Nature. 1997; 389:149–152. PMID: 9296491.
Article22. Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001; 154:925–936. PMID: 11535616.
Article23. King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994; 79:563–571. PMID: 7954823.
Article24. Hubscher U, Nasheuer HP, Syvaoja JE. Eukaryotic DNA polymerases, a growing family. Trends Biochem Sci. 2000; 25:143–147. PMID: 10694886.25. Katsumi S, Kobayashi N, Imoto K, Nakagawa A, Yamashina Y, Muramatsu T, Shirai T, Miyagawa S, Sugiura S, Hanaoka F, Matsunaga T, Nikaido O, Mori T. In situ visualization of ultraviolet- light-induced DNA damage repair in locally irradiated human fibroblasts. J Invest Dermatol. 2001; 117:1156–1161. PMID: 11710927.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Gene Expression Profile Using cDNA microarray after treatment Arsenic Compound (As2O3, As4O6) in SiHa Cell
- Tetraarsenic Oxide-mediated Apoptosis in a Cervical Cancer Cell Line, SiHa
- Retraction: Comparison of As2O3 and As4O6 in the Detection of SiHa Cervical Cancer Cell Growth Inhibition Pathway
- Vascular shutdown effects by tetraarsenic oxide in TC-1 cells implanted C57BL/6 mice
- Development of Gene Therapy Strategy Using Plasmid and Adenovirus in Cervical Cancer Treatment