Cancer Res Treat.
2004 Aug;36(4):228-234.
Efficacy of Postoperative Concurrent Chemoradiation for Resectable Rectal Cancer: A Single Institute Experience
- Affiliations
-
- 1Center for Colorectal Cancer, National Cancer Center, Korea.
- 2Department of Internal Medicine, Yonsei University College of Medicine, Korea. unchung8@yumc.yonsei.ac.kr
- 3Department of General Surgery, Yonsei University College of Medicine, Korea.
- 4Department of Radiation Oncology, Yonsei University College of Medicine, Korea.
- 5Yonsei Cancer Center, Korea.
- 6Brain Korea 21 Project for Medical Science, Korea.
Abstract
- PURPOSE
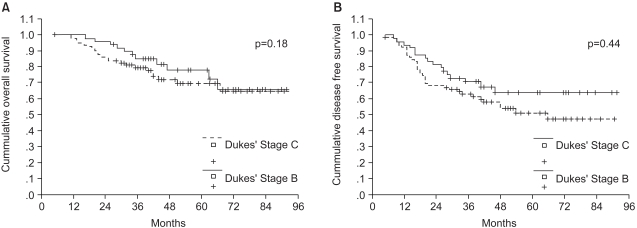
For patients with Dukes' stage B and C rectal cancer, surgery followed by adjuvant chemoradiotherapy is considered to be the standard treatment. However, the drugs used in combination with 5-fluorouracil (5-FU), the method of administration, duration of adjuvant therapy and the frequencies of administration presently remain controversial topics. We investigated (1) the efficacy and safety of adjuvant radiotherapy and 5-FU/leucovorin (LV) chemotherapy for patients who had undergone curative resection and (2) the effect of dose related factors of 5-FU on survival. MATERIALS AND METHODS: 130 rectal cancer patients with Dukes' B or C stage disease who were treated with curative resection were evaluated. The adjuvant therapy consisted of two cycles of 5-FU/LV chemotherapy followed by pelvic radiotherapy with chemotherapy, and then 4~10 more cycles of the same chemotherapy regimen were delivered based on the disease stage. The cumulative dose of 5-FU per body square meter (BSA), actual dose intensity and relative dose intensity were obtained. The patients were divided into two groups according to the median value of each factor, and the patients' survival rates were compared. RESULTS: With a median follow-up duration of 52 months, the 5-year disease-free survival and overall survival rates of 130 patients were 57% and 73%, respectively. Loco- regional failure occurred in 17 (13%) of the 130 patients, and the distant failure rate was 27% (35/130). The chemotherapy related morbidity was minimal, and there was no mortality for these patients. The cumulative dose of 5-FU/ BSA had a significant effect on the 5-year overall survival for Dukes' C rectal cancer patients (p=0.03). Multivariate analysis demonstrated that only the performance status affected the 5-year overall survival (p=0.003). CONCLUSION: An adjuvant therapy of radiotherapy and 5-FU/LV chemotherapy is effective and tolerable for Dukes' B and C rectal cancer patients. A rospective, multicenter, randomized study to evaluate the effects of the cumulative dose of 5-FU/BSA on survival is required.
MeSH Terms
Figure
Reference
-
1. Mohiuddin M, Marks G. Adjuvant radiation therapy of colon and rectal cancer. Semin Oncol. 1991; 8:411–420. PMID: 1925630.2. Pilipshen SJ, Heilweil M, Sternberg SH, Sternberg SS, Enker WE. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer. 1984; 53:1354–1362. PMID: 6692324.
Article3. Mendenhall MW, Million RR, Pfaff WW. Patterns of recurrence in adenocarcinoma of the rectum and rectosigmoid traeted with surgery alone: Implications in treatment planning with adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 1983; 9:977–985. PMID: 6863077.4. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically resected rectal adenocarcinoma. N Engl J Med. 1985; 312:1465–1472. PMID: 2859523.5. Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, WIchkerham DL, Fisher ER, Caplan R, Jones J, Lerner H, Gordon P, Feldman M, Cruz A, Legault-Poisson S, Welxler M, Lawrence W, Robidoux A. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer. J Natl Cancer Inst. 1988; 80:21–29. PMID: 3276900.6. Gerard A, Buyse M, Nordlinger B, Loygue J, Pene F, Kempf P, Arnaud JP, Desaive C, Duez N. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg. 1988; 211:606–614. PMID: 3056288.7. Higgins GA. Current status of adjuvant therapy in the treatment of large bowel tumor. Surg Clin North Am. 1983; 63:137–150. PMID: 6338606.8. Byfield JE, Calabro-Jones P, Klisak I. Pharmacologic requirements human tumor cells in vitro to combined 5-fluorouracil or ftorafur and x-rays. Int J Radiat Oncol Biol Phys. 1982; 8:1923–1933. PMID: 6818194.9. Keyomarsi K, Moran RG. Folinic acid augmentation of the effects of fluoropyrimidines on murine and human leukemia cells. Cancer Res. 1986; 46:5229–5235. PMID: 2944577.10. Madajewicz S, Petrelli N, Rustum YM, Campbell J, Herrera L, Mittelman A, Perry A, Creaven PJ. Phase I-II trial of high dose calcium leucovorin and 5-fluorouracil in advanced colorectal cancer. Cancer Res. 1984; 44:4667–4669. PMID: 6331882.11. Machover D, Goldschmidt E, Chollet P, Metzger G, Zittoun J, Marguet J, Vandenbulcke JM, Misset JL, Schwarzenbergh L, Fourtillan JB, Gaget H, Mathe G. Treatment of advanced colorectal and gastric adenocarcinoma with 5-fluorouracil and high dose folinic acid. J Clin Oncol. 1986; 4:685–696. PMID: 3517242.12. NIH Consensus Conference. Adjuvant Therapy for Patients With Colon and Rectal Cancer. JAMA. 1990; 264:1444–1450. PMID: 2202842.13. Buyse M, Zeleniuch-Jacquotte A. Adjuvant therapy of colorectal cancr. Why we still don't know. JAMA. 1988; 259:3571–3578. PMID: 3286920.14. Moertel CG. Chemotherapy for colorectal cancer. N Engl J Med. 1994; 330:1136–1142. PMID: 8133857.
Article15. Balslev IB, Pederson M. Postoperative radiotherapy in Dukes' B and C carcinoma of the rectum and rectosigmoid. Cancer. 1986; 58:22–28. PMID: 3518912.16. Yoo MR, Jang HS, Yoon SC, Chung SM, Kim YS, Kim SK, Kim IC, Shin KS. The Results of Postoperative Radiation Therapy in the Rectal Cancer. J Korean Cancer Assoc. 1997; 29:111–116.17. Krook JE, Moertel CG, Gunderson LL. Effective Surgical Adjuvant Therapy for High-Risk Rectal Carcinoma. N Engl J Med. 1991; 324:709–715. PMID: 1997835.
Article18. O'Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994; 331:502–507. PMID: 8041415.19. Smalley SR, Benedetti J, Williamson S, Robertson J, Fisher B, Martenson J, Benson AB, Robert M, Cripps C, MacDonald J. Intergroup 0144 - phase III trial of 5-FU based chemotherapy regimens plus radiotherapy (XRT) in postoperative adjuvant rectal cancer. Bolus 5-FU vs prolonged venous infusion (PVI) before and after XRT+PVI vs bolus 5-FU+ leucovorin (LV)+levamisole (LEV) before and after XRT+ bolus 5-FU+LV. Proc Am Soc Clin Oncol. 2003; 22:251. (abstr 1006).20. Rodriguez-Bigas MA, Lin EH, Crane CH. Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, Frei E, editors. Adenocarcinoma of the colon and rectum. Cancer Medicine. 2003. Vol 2:6th ed. Hamilton: BC Decker;p. 1635–1665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Outcome of Preoperative Chemoradiation to Locally Advanced Rectal Cancer
- Disadvantages of Preoperative Chemoradiation in Rectal Cancer
- Late Complications after Preoperative Chemoradiation for Rectal Cancers
- Capecitabine-based Neoadjuvant Chemoradiation Therapy in Locally-advanced Rectal Cancer
- Selective Approach to Sphincter-Saving Procedure after Chemoradiation in Low Rectal Cancer