Cancer Res Treat.
2004 Dec;36(6):384-388.
Expression of Matrix Metalloproteinase-9 Correlates with Poor Prognosis in Human Malignant Fibrous Histiocytoma
- Affiliations
-
- 1Department of Pathology, St. Vincent's Hospital, The Catholic University of Korea, Suwon, Korea. apjjh225@catholic.ac.kr
Abstract
- PURPOSE
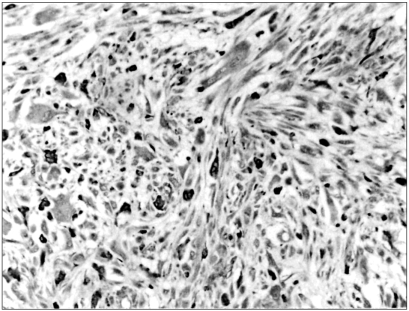
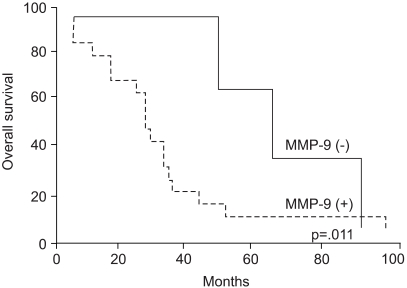
92 kDa matrix metalloproteinase-9 (MMP-9) is believed to play an important role in degrading the matrix and basement membrane, contributing to the invasion and metastasis of malignant solid tumors. However, little is known about its involvement in a malignant fibrous histiocytoma. The aim of this study was to investigate the expression of MMP-9 and to correlate its expression with clinicopathologic parameters in human malignant fibrous histiocytomas. MATERIALS AND METHODS: Archival tumor tissues from 20 patients with a malignant fibrous histiocytoma were analyzed by immunohistochemistry for the expression of MMP-9. Clinical information was obtained through the computerized retrospective database from the tumorregistry. RESULTS: Seventeen of 20 (85%) tumors showed a positive reaction for MMP-9. MMP-9 activity was inversely correlated with patients' survival time (p=.011). There was no significant correlation between the activated MMP-9 expression and all the other clinicopathologic variables. CONCLUSION: Our data demonstrate that MMP-9 activation is likely to occur in human malignant fibrous histiocytomas. It is also noteworthy that the expression of MMP-9 may aid in predicting patients' poor prognosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Pisters PWT, Brennan MF. Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Sarcomas of soft tissue. Clinical Oncology. 2000. 2nd ed. New York: Churchill Livingstones;p. 2273–2313.2. Enzinger F, Weiss S. Soft Tissue Tumors. 1995. 3rd ed. St. Louis: Mosby.3. Pezzi CM, Rawlings MS Jr, Esgro JJ, Pollock RE, Romsdahl MM. Prognostic factors in 227 patients with malignant fibrous histiocytoma. Cancer. 1992; 69:2098–2103. PMID: 1311983.
Article4. Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor of cell interactions with the extracellular matrix during invasion and metastasis. Ann Rev Cell Biol. 1993; 9:541–573. PMID: 8280471.5. Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999; 43(Suppl):S42–S51. PMID: 10357558.
Article6. Scappaticci FA, Marina N. New molecular targets and biological therapies in sarcomas. Cancer Treat Rev. 2001; 27:317–326. PMID: 11908925.
Article7. Sanceau J, Boyd DD, Seiki M, Bauvois B. Interferons inhibit tumor necrosis factor-α-mediated matrix metalloproteinase-9 activation via interferon regulatory factor-1 binding competition with NF-κB. J Biol Chem. 2002; 277:35766–35775. PMID: 12105194.8. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000; 18:1135–1149. PMID: 10694567.
Article9. Bauvois B, Dumont J, Mathiot C, Kolb JP. Production of matrix metalloproteinase-9 in early stage B-CLL: suppression by interferons. Leukemia. 2002; 16:791–798. PMID: 11986939.
Article10. Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of MMP-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ. 1999; 10:353–367. PMID: 10359016.11. Coindre JM. Grading in soft tissue sarcomas-how and why? Histopathology. 2002; 41(Suppl 2):236–240. PMID: 12207785.12. Yoo J, Jung JH, Choi HJ, Kang SJ, Kang CS. The expression of c-myc, bcl-2 and p53 proteins in adenocarcinomas of lung. Cancer Res Treat. 2002; 36:146–150.
Article13. Soini Y, Salo T, Oikarinen A, Autio-Harmainen H. Expression of 72 and 92 kDa type IV collagenase in malignant fibrous histiocytomas and dermatofibromas. Lab Invest. 1993; 69:305–311. PMID: 8397322.14. Ohnishi Y, Ito Y, Tajima S, Ishibashi A, Arai K. Immunohistochemical study of membrane type-matrix metalloproteinases (MT-MMPs) and matrix metalloproteinase-2 (MMP-2) in dermatofibroma and malignant fibrous histiocytoma. J Dermatol Sci. 2002; 28:119–125. PMID: 11858950.
Article15. Reynolds JJ. Collagenases and tissue inhibitor of metalloproteinases: A functional balance in tissue degradation. Oral Dis. 1996; 2:70–76. PMID: 8957940.16. McMillan JI, Weeks R, West JW, Bursten S, Rice GC, Lovett DH. Pharmacological inhibition of gelatinase B induction and tumor cell invasion. Int J Cancer. 1996; 67:523–531. PMID: 8759612.
Article17. Brown PD. Parks WC, Mecham RP, editors. Synthetic inhibitors of matrix metalloproteinases. Matrix metalloproteinases. 1998. Sandieqo: Academic Press;p. 243–256.
Article18. Hua J, Muschel RJ. Inhibition of matrix metalloproteinase-9 expression by a ribozyme blocks metastasis in a rat sarcoma model system. Cancer Res. 1996; 56:5279–5284. PMID: 8912869.19. Heikkila P, Teronen O, Hirn MY, Sorsa T, Tervahartiala T, Salo T, et al. Inhibition of matrix metalloproteinase-14 in osteosarcoma cells by clodronate. J Surg Res. 2003; 111:45–52. PMID: 12842447.20. Fingleton B, Matrisian LM. Matrix metalloproteinsases as targets for therapy in Kaposi sarcoma. Curr Opin Oncol. 2001; 13:368–373. PMID: 11555714.21. Foukas AF, Deshmukh NS, Grimer RJ, Mangham DC, Mangos EG, Taylor S. Stage-IIB osteosarcomas around the knee. A study of MMP-9 in surviving tumour cells. J Bone Joint Surg. 2002; 84:706–711.22. Himmelstein BP, Asada N, Carlton MR, Collins MH. Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediat Oncol. 1998; 31:471–474.23. Rao JS, Yamamoto N, Mohaman S, Gokaslan ZL, Fuller ZN, Stetler-Stevenson WG, et al. Expression and localization of 92-kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996; 14:12–18. PMID: 8521611.24. MacDougall JR, Bani MR, Lin Y, Rak J, Kerbel RS. The 92-kDa gelatinase B is expressed by advanced stage melanoma cells: suppression by somatic cell hybridization with early stage melanoma cells. Cancer Res. 1995; 55:4174–4181. PMID: 7664294.25. Papathoma AS, Petraki C, Grigorakis A, Papakonstantinou H, Karavana V, Stefanakis S, et al. Prognostic significance of matrix metalloproteinases 2 and 9 in bladder cancer. Anticancer Res. 2000; 20:2009–2013. PMID: 10928143.