Cancer Res Treat.
2005 Oct;37(5):307-312.
Tetraarsenic Oxide-mediated Apoptosis in a Cervical Cancer Cell Line, SiHa
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Catholic University of Korea College of Medicine, Korea. ahnws@catholic. ac.kr
- 2Cancer Research Center, Catholic Research Institutes of Medical Science, The Catholic University of Korea College of Medicine, Korea.
- 3Laboratory of Chonjisan Institute, Seoul, Korea.
- 4Department of Pathology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 5Department of Bioscience and Biotechnology, Institute of Biotechnology, College of Life Science, Sejong University, Seoul, Korea.
- 6College of Pharmacy, Seoul National University, Seoul, Korea.
Abstract
- PURPOSE
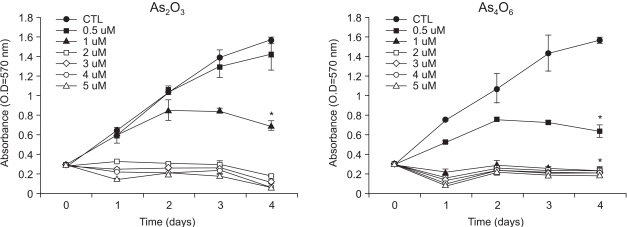
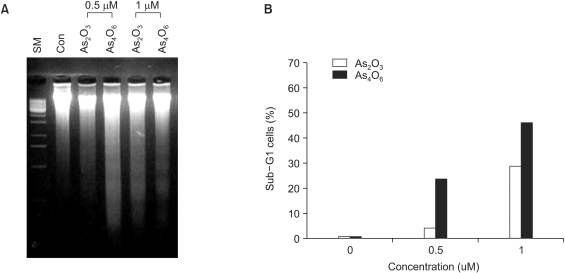
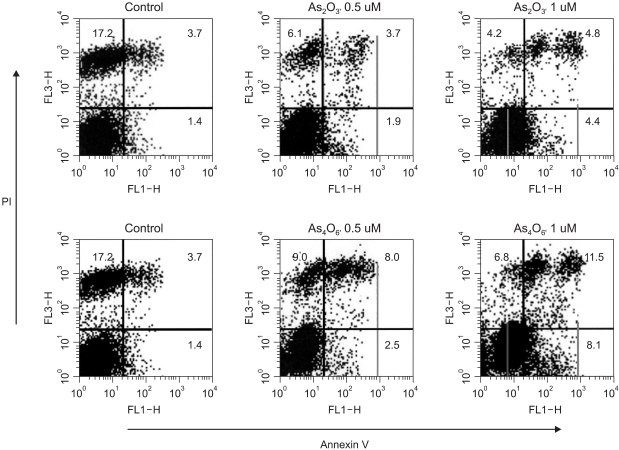
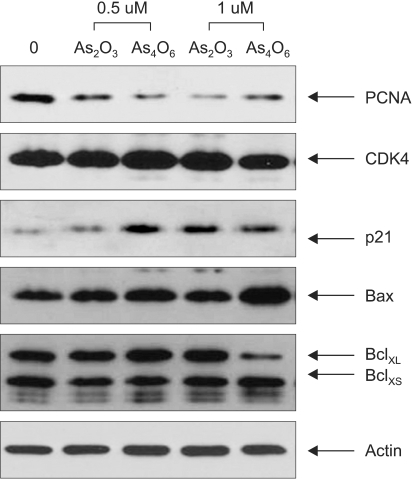
Diarsenic oxide, As2O3, has been reported to be effective in treating acute leukemia, and induce apoptosis in many tumor cells. In this study, the ability of a novel arsenical compound, As4O6 (tetraarsenic oxide), along with As2O3, for its ability to induce cell growth inhibition, as well as apoptosis, in human cervical cancer cells, SiHa cells, were evaluated in vitro. MATERIALS AND METHODS: To examine the levels of apoptosis, SiHa cells were given two sensitive doses, 0.5 and 1micrometer, of arsenical compounds, and a DNA fragmentation assay and FACS analysis were then conducted. In addition, a Western blotting assay was performed to identify target molecules for apoptosis. RESULTS: Both As2O3 and As4O6 induced dose-dependent inhibition of SiHa cell proliferation. In particular, As4O6 was more effective at suppressing SiHa cell growth than As2O3. In parallel with the inhibition of cell proliferation, As4O6 caused a significantly greater increase in the sub-G1 cell population than As2O3, as determined by propidium iodide DNA staining. This was confirmed by a DNA fragmentation assay and annexin V staining. The Western blotting analysis also showed that the expression of proliferating cell nuclear antigen (PCNA) was suppressed to a significantly greater extent by As4O6 than As2O3 at a dose of 0.5micrometer. However, the apoptosis-related protein, Bax, was expressed to a significantly greater extent due to As4O6 than As2O3. CONCLUSIONS: Taken together, these findings suggest that a novel arsenic compound, As4O6, possesses more potent anti-proliferative effects on human cervical cancer cells, with the induction of apoptosis also, at least via the activation of Bax protein in vitro.
Keyword
MeSH Terms
Figure
Reference
-
1. Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997; 89:3354–3360. PMID: 9129042.2. Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti L, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998; 339:1341–1348. PMID: 9801394.
Article3. Zhang P, Wang SY, Hu LH, Shi FD, Qiu FD, Hong RJ, et al. Arsenic trioxide treated 72 cases of acute promyelocytic leukemia. Chi J Hematol. 1996; 17:58–70.4. Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-PARalpha/PML proteins. Blood. 1996; 88:1052–1061. PMID: 8704214.5. Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000; 60:3065–3071. PMID: 10850458.6. Gurr JR, Bau DT, Liu F, Lynn S, Jan KY. Dithiothreitol enhances arsenic trioxide-induced apoptosis in NB4 cells. Mol Pharmacol. 1999; 56:102–109. PMID: 10385689.
Article7. Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA- immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer. 1999; 82:286–292. PMID: 10389765.8. Li X, Ding X, Adrian TE. Arsenic trioxide inhibits proliferation and induces apoptosis in pancreatic cancer cells. Anticancer Res. 2002; 22:2205–2213. PMID: 12174905.9. Li X, Ding X, Adrian TE. Arsenic trioxide induces apoptosis in pancreatic cancer cells via changes in cell cycle, caspase activation and GADD expression. Pancreas. 2003; 27:174–179. PMID: 12883267.
Article10. Cullen AP, Reid R, Campion M, Lorincz AT. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991; 65:606–612. PMID: 1846186.
Article11. Lorincz AT, Temple GF, Kurman RJ, Jenson AB, Lancaster WD. Oncogenic association of specific papillomavirus types with cervical neoplasia. J Natl Cancer Inst. 1987; 79:671–677. PMID: 2821311.12. zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989; 49:4677–4681. PMID: 2547512.13. Scheffner M, Werness BA, Heibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990; 63:1129–1136. PMID: 2175676.
Article14. Scheffner M, Munger K, Bryne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991; 88:5523–5527. PMID: 1648218.
Article15. Werness BA, Levine AJ, Howley PM. Association of HPV type 16 and 18 E6 protein with p53. Science. 1990; 248:76–79. PMID: 2157286.16. Park IC, Park MJ, Woo SH, Lee HC, An S, Gwak HS, et al. Tetraarsenic oxide induces apoptosis in U937 leukemic cells through a reactive oxygen species-dependent pathway. Int J Oncol. 2003; 23:943–948. PMID: 12963972.
Article17. Park MJ, Park IC, Bae IJ, Seo KM, Lee SH, Hong SI, et al. Tetraarsenic oxide, a novel orally administrable angiogenesis inhibitor. Int J Oncol. 2003; 22:1271–1276. PMID: 12738993.
Article18. Ahn WS, Bae SM, Lee KH, Kim YW, Lee JM, Namkoong SE, et al. Comparison of effects of As2O3 and As4O6 on cell growth inhibition and gene expression profiles by cDNA microarray analysis in SiHa cells. Oncol Rep. 2004; 12:573–580. PMID: 15289840.19. Yoo MH, Kim JT, Rhee CH, Park MJ, Bae IJ, Yi NY, et al. Reverse effects of tetraarsenic oxide on the angiogenesis induced by nerve growth factor in the rat cornea. J Vet Med Sci. 2004; 66:1091–1095. PMID: 15472473.
Article20. Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999; 93:268–277. PMID: 9864170.
Article21. Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the national cancer institute cooperative research and development studies. Oncologist. 2001; 6(Suppl. 2):22–28. PMID: 11331437.
Article22. Astudillo H, Lopez T, Castillo S, Gariglio P, Benitez L. p53, Bcl-2, PCNA expression, and apoptotic rates cervical tumorigenesis. Ann N Y Acad Sci. 2003; 1010:771–774. PMID: 15033825.23. Mitchell KO, Ricci MS, Miyashita T, Dicker DT, Jin Z, Reed JC, et al. Bax is a transcriptional target and mediator of c-myc-induced apoptosis. Cancer Res. 2000; 60:6318–6325. PMID: 11103792.24. St. John LS, Sauter ER, Herlyn M, Litwin S, Adler-Storthz K. Endogenous p53 gene status predicts the response of human squamous cell carcinoma to wild-type p53. Cancer Gene Ther. 2000; 7:749–756. PMID: 10830722.25. Takehara T, Takahashi H. Suppression of Bcl-XL deamidation in human hepatocellular carcinomas. Cancer Res. 2003; 63:3054–3057. PMID: 12810626.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of cell growth and apoptosis in CaSki, cervical cancer cell line by arsenic compounds
- Retraction: Tetraarsenic Oxide-mediated Apoptosis in a Cervical Cancer Cell Line, SiHa
- Antitumor Effects of Camptothecin Combined with Conventional Anticancer Drugs on the Cervical and Uterine Squamous Cell Carcinoma Cell Line SiHa
- Cell Cycle Regulatory Protein Expression Profiles by Adenovirus p53 Infection in Human Papilloma Virus-associated Cervical Cancer Cells
- Changes of Gene Expression between HaCat and SiHa cells using GeneFishingTM PCR