Cancer Res Treat.
2005 Dec;37(6):319-324.
The Role of HPV E6 and E7 Oncoproteins in HPV-associated Cervical Carcinogenesis
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Catholic University of Korea College of Medicine, Seoul, Korea. jspark@catholic.ac.kr
Abstract
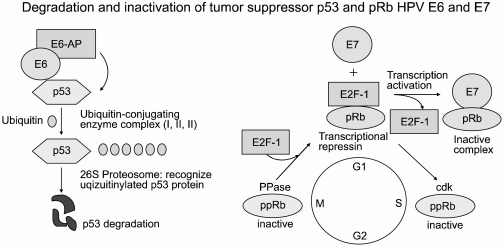
- Cervical cancer is one of the leading world causes of cancer morbidity and mortality in woman, with more than 98% related to a human papillomavirus (HPV) infection origin. Infection with specific subtypes of HPV has been strongly implicated in cervical carcinogenesis. The identification and functional verification of host proteins associated with HPV E6 and E7 oncoproteins may provide useful information in understanding cervical carcinogenesis and the development of cervical cancer-specific markers. The advent of functional genomics and proteomics has provided hope of discovering novel biological markers for use in the screening, early diagnosis, prognostication and prediction of response to therapy. Herein, we review the studies where the profiles of host proteins associated with HPV E6 and E7 oncoproteins in cervical cancer were generated.
Keyword
MeSH Terms
Figure
Reference
-
1. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12–19. PMID: 10451482.
Article2. Lipari F, McGibbon GA, Wardrop E, Cordingley MG. Purification and biophysical characterization of a minimal functional domain and of an N-terminal Zn2+-binding fragment from the human papillomavirus type 16 E6 protein. Biochemistry. 2001; 40:1196–1204. PMID: 11170444.3. Crook T, Tidy JA, Vousden KH. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell. 1991; 67:547–556. PMID: 1657399.
Article4. Jones DL, Munger K. Interactions of the human papillomavirus E7 protein with cell cycle regulators. Semin Cancer Biol. 1996; 7:327–337. PMID: 9284525.
Article5. Lipinski MM, Jacks T. The retinoblastoma gene family in differentiation and development. Oncogene. 1999; 18:7873–7882. PMID: 10630640.
Article6. Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, et al. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992; 89:4549–4553. PMID: 1316611.
Article7. Cheng S, Schmidt-Grimminger DC, Murant T, Broker TR, Chow LT. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995; 9:2335–2349. PMID: 7557386.
Article8. Filippova M, Parkhurst L, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to Fas-associated death domain and protects cells from Fas-triggered apoptosis. J Biol Chem. 2004; 279:25729–25744. PMID: 15073179.
Article9. Yim EK, Meoyng J, Namakoong SE, Um SJ, Park JS. Genomic and proteomic expression patterns in HPV-16 E6 gene transfected stable human carcinoma cell lines. DNA Cell Biol. 2004; 23:826–835. PMID: 15684709.
Article10. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990; 63:1129–1136. PMID: 2175676.
Article11. Degenhardt K, Sundararajan R, Lindsten T, Thompson C, White E. Bax and Bak independently promote cytochrome C release from mitochondria. J Biol Chem. 2002; 277:14127–14134. PMID: 11836241.12. Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001; 8:705–711. PMID: 11583631.
Article13. Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996; 383:443–446. PMID: 8837778.
Article14. Kaufmann WK, Schwartz JL, Hurt JC, Byrd LL, Galloway DA, Levedakou E, et al. Inactivation of G2 checkpoint function and chromosomal destabilization are linked in human fibroblasts expressing human papillomavirus type 16 E6. Cell Growth Differ. 1997; 8:1105–1114. PMID: 9342189.15. Park JS, Kim EJ, Lee JY, Sin HS, Namkoong SE, Um SJ. Functional inactivation of p73, a homolog of p53 tumor suppressor protein, by the human papillomavirus E6 proteins. Int J Cancer. 2001; 91:822–827. PMID: 11275986.16. Yim EK, Um SJ, Park JS. Interaction of HPV E6 oncoprotein with the novel protein, BARD1 (BRCA1-associated ring domain 1) and its biological role. 2005. In : 22nd Intl. Papillomavirus Conference;17. Park JS, Kim EJ, Kwon HJ, Hwang ES, Namkoong SE, Um SJ. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein; implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000; 275:6764–6769. PMID: 10702232.18. Barnard P, McMillan NA. The human papillomavirus E7 oncoprotein abrogates signaling mediated by interferon-alpha. Virology. 1999; 259:305–313. PMID: 10388655.19. Lee KA, Shim JH, Kho CW, Park SG, Park BC, Kim JW, et al. Protein profiling and identification of modulators regulated by the E7 oncogene in the C33A cell line by proteomics and genomics. Proteomics. 2004; 4:839–848. PMID: 14997504.
Article20. Versteege I, Medjkane S, Rouillard D, Delattre O. A key role of the hSNF5/INI1 tumour suppressor in the control of the G1-S transition of the cell cycle. Oncogene. 2002; 21:6403–6412. PMID: 12226744.
Article21. Lee D, Kim JW, Seo T, Hwang SG, Choi EJ, Choe J. SWI/SNF complex interacts with tumor suppressor p53 and is necessary for the activation of p53-mediated transcription. J Biol Chem. 2002; 277:22330–22337. PMID: 11950834.
Article22. Tokunaga E, Maehara Y, Oki E, Kitamura K, Kakeji Y, Ohno S, et al. Diminished expression of ING1 mRNA and the correlation with p53 expression in breast cancers. Cancer Lett. 2000; 152:15–22. PMID: 10754201.
Article23. Lee KA, Kang JW, Shim JH, Kho CW, Park SG, Lee HG, et al. Protein profiling and identification of modulators regulated by human papillomavirus 16 E7 oncogene in HaCaT keratinocytes by proteomics. Gynecol Oncol. 2005; 99:142–152. PMID: 16038965.
Article24. Tremblay GM, Janelle MF, Bourbonnais Y. Anti-inflammatory activity of neutrophil elastase inhibitors. Curr Opin Investig Drugs. 2003; 4:556–565.25. Kimura E, Enns RE, Thiebaut F, Howell SB. Regulation of HSP60 mRNA expression in a human ovarian carcinoma cell line. Cancer Chemother Pharmacol. 1993; 32:279–285. PMID: 8100743.
Article26. Yokota S, Yanagi H, Yura T, Kubota H. Cytosolic chaperonin is up-regulated during cell growth Preferential expression and binding to tubulin at G(1)/S transition through early S phase. J Biol Chem. 1999; 274:37070–37078. PMID: 10601265.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Papillomavirus Infection
- Natural history of HPV and carcinogenesis of cervical cancer
- Integration of HPV and the Antibody Respones to HPV Proteins in Patients with Cervical Cancer
- B Cells Transduced with HPV16 E6/E7-expressing Adenoviral Vector Can Efficiently Induce CTL-dependent Anti-Tumor Immunity
- A study on expression of Human Papillomavirus 16/18, E6/ E7 and Ki-67 in the cervical cancer